Canada: These 76 Drugs Face Patent Expirations and Generic Entry in 2025 - 2026
DrugPatentWatch® Estimated Loss of Exclusivity Dates in Canada
Generic Entry Dates in Other Countries
These estimated drug patent expiration dates and generic entry opportunity dates are calculated from analysis of known patents covering drugs. Many factors can influence early or late generic entry. This information is provided as a rough estimate of generic entry potential and should not be used as an independent source. The methodology is described in this blog post.
Summary: Canada: These 76 Drugs Face Patent Expirations and Generic Entry in 2025 - 2026
| Tradename | Ingredient | Estimated Entry Opportunity Date |
|---|---|---|
| CREON | pancrelipase (amylase;lipase;protease) | 2026-08-15 |
| EMBEDA | morphine sulfate; naltrexone hydrochloride | 2026-06-19 |
| AMITIZA | lubiprostone | 2026-01-24 |
| >Tradename | >Ingredient | >Estimated Entry Opportunity Date |
Details: Canada: These 76 Drugs Face Patent Expirations and Generic Entry in 2025 - 2026
When can CREON (pancrelipase (amylase;lipase;protease)) generic drug versions launch?
Generic name: pancrelipase (amylase;lipase;protease)
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 15, 2026
Generic Entry Controlled by: Canada Patent 2,619,475
CREON is a drug marketed by
See drug price trends for CREON.
The generic ingredient in CREON is pancrelipase (amylase;lipase;protease). There are six drug master file entries for this API. Additional details are available on the pancrelipase (amylase;lipase;protease) profile page.
When can CREON (pancrelipase (amylase;lipase;protease)) generic drug versions launch?
Generic name: pancrelipase (amylase;lipase;protease)
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 15, 2026
Generic Entry Controlled by: Canada Patent 2,619,477
CREON is a drug marketed by
See drug price trends for CREON.
The generic ingredient in CREON is pancrelipase (amylase;lipase;protease). There are six drug master file entries for this API. Additional details are available on the pancrelipase (amylase;lipase;protease) profile page.
When can EMBEDA (morphine sulfate; naltrexone hydrochloride) generic drug versions launch?
Generic name: morphine sulfate; naltrexone hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 19, 2026
Generic Entry Controlled by: Canada Patent 2,655,835
EMBEDA is a drug marketed by Alpharma Pharms. There are nine patents protecting this drug and four Paragraph IV challenges.
This drug has seventy-four patent family members in twenty-three countries.
See drug price trends for EMBEDA.
The generic ingredient in EMBEDA is morphine sulfate; naltrexone hydrochloride. There are twenty-three drug master file entries for this API. Additional details are available on the morphine sulfate; naltrexone hydrochloride profile page.
When can AMITIZA (lubiprostone) generic drug versions launch?
Generic name: lubiprostone
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: January 24, 2026
Generic Entry Controlled by: Canada Patent 2,637,274
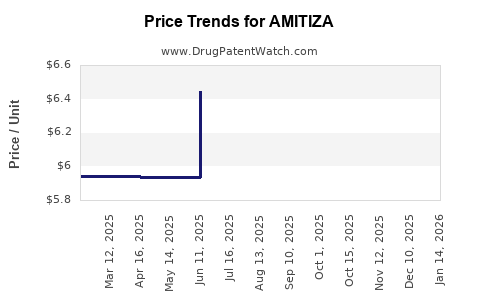
This drug has forty-nine patent family members in nineteen countries. There has been litigation on patents covering AMITIZA
See drug price trends for AMITIZA.
The generic ingredient in AMITIZA is lubiprostone. There are ten drug master file entries for this API. Twenty suppliers are listed for this generic product. Additional details are available on the lubiprostone profile page.
When can ESBRIET (pirfenidone) generic drug versions launch?
Generic name: pirfenidone
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: September 22, 2026
Generic Entry Controlled by: Canada Patent 2,620,380
ESBRIET is a drug marketed by Genentech Inc. There are twenty patents protecting this drug and two Paragraph IV challenges. One tentatively approved generic is ready to enter the market.
This drug has two hundred and sixty-six patent family members in forty-six countries. There has been litigation on patents covering ESBRIET
See drug price trends for ESBRIET.
The generic ingredient in ESBRIET is pirfenidone. There are twenty-three drug master file entries for this API. Twenty-four suppliers are listed for this generic product. Additional details are available on the pirfenidone profile page.
When can LYNPARZA (olaparib) generic drug versions launch?
Generic name: olaparib
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: October 17, 2026
Generic Entry Controlled by: Canada Patent 2,664,275
LYNPARZA is a drug marketed by Astrazeneca. There are twelve patents protecting this drug. Three tentatively approved generics are ready to enter the market.
This drug has two hundred and fifty-four patent family members in fifty-two countries. There has been litigation on patents covering LYNPARZA
See drug price trends for LYNPARZA.
The generic ingredient in LYNPARZA is olaparib. There are three drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the olaparib profile page.
When can OLYSIO (simeprevir sodium) generic drug versions launch?
Generic name: simeprevir sodium
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: July 28, 2026
Generic Entry Controlled by: Canada Patent 2,616,580
OLYSIO is a drug marketed by Janssen Prods. There are nine patents protecting this drug.
This drug has one hundred and forty patent family members in forty-three countries.
See drug price trends for OLYSIO.
The generic ingredient in OLYSIO is simeprevir sodium. There is one drug master file entry for this API. Additional details are available on the simeprevir sodium profile page.
When can XALKORI (crizotinib) generic drug versions launch?
Generic name: crizotinib
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: November 23, 2026
Generic Entry Controlled by: Canada Patent 2,632,283
XALKORI is a drug marketed by Pf Prism Cv. There are five patents protecting this drug.
This drug has one hundred and fifty-two patent family members in forty-eight countries.
See drug price trends for XALKORI.
The generic ingredient in XALKORI is crizotinib. One supplier is listed for this generic product. Additional details are available on the crizotinib profile page.
When can PICATO (ingenol mebutate) generic drug versions launch?
Generic name: ingenol mebutate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: December 18, 2026
Generic Entry Controlled by: Canada Patent 2,634,073

This drug has thirty-five patent family members in twenty-one countries. There has been litigation on patents covering PICATO
See drug price trends for PICATO.
The generic ingredient in PICATO is ingenol mebutate. There are three drug master file entries for this API. Additional details are available on the ingenol mebutate profile page.
When can LASTACAFT (alcaftadine) generic drug versions launch?
Generic name: alcaftadine
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: March 31, 2026
Generic Entry Controlled by: Canada Patent 2,648,115
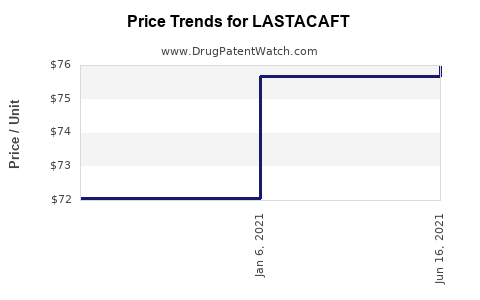
This drug has forty-six patent family members in thirty countries. There has been litigation on patents covering LASTACAFT
See drug price trends for LASTACAFT.
The generic ingredient in LASTACAFT is alcaftadine. There are six drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the alcaftadine profile page.
When can AXUMIN (fluciclovine f-18) generic drug versions launch?
Generic name: fluciclovine f-18
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: November 28, 2026
Generic Entry Controlled by: Canada Patent 2,629,227
AXUMIN is a drug marketed by Blue Earth. There are eight patents protecting this drug.
This drug has thirty patent family members in sixteen countries. There has been litigation on patents covering AXUMIN
The generic ingredient in AXUMIN is fluciclovine f-18. One supplier is listed for this generic product. Additional details are available on the fluciclovine f-18 profile page.
When can LEXISCAN (regadenoson) generic drug versions launch?
Generic name: regadenoson
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 03, 2026
Generic Entry Controlled by: Canada Patent 2,640,089
LEXISCAN is a drug marketed by Astellas. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has twenty-eight patent family members in eighteen countries. There has been litigation on patents covering LEXISCAN
See drug price trends for LEXISCAN.
The generic ingredient in LEXISCAN is regadenoson. There are nine drug master file entries for this API. Eighteen suppliers are listed for this generic product. Additional details are available on the regadenoson profile page.
When can VANTRELA ER (hydrocodone bitartrate) generic drug versions launch?
Generic name: hydrocodone bitartrate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: September 15, 2026
Generic Entry Controlled by: Canada Patent 2,699,142
VANTRELA ER is a drug marketed by Teva Branded Pharm. There are three patents protecting this drug. One tentatively approved generic is ready to enter the market.
This drug has thirty-three patent family members in thirteen countries. There has been litigation on patents covering VANTRELA ER
The generic ingredient in VANTRELA ER is hydrocodone bitartrate. There are twenty-three drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the hydrocodone bitartrate profile page.
When can APTIOM (eslicarbazepine acetate) generic drug versions launch?
Generic name: eslicarbazepine acetate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: April 21, 2026
Generic Entry Controlled by: Canada Patent 2,616,984
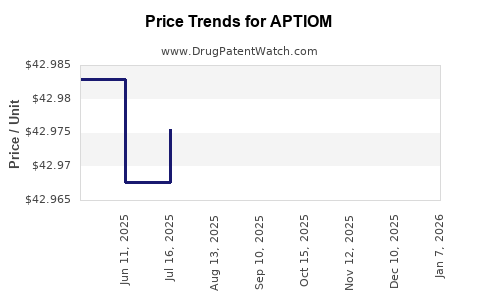
This drug has one hundred patent family members in twenty-six countries. There has been litigation on patents covering APTIOM
See drug price trends for APTIOM.
The generic ingredient in APTIOM is eslicarbazepine acetate. There are twelve drug master file entries for this API. Eight suppliers are listed for this generic product. Additional details are available on the eslicarbazepine acetate profile page.
When can BOSULIF (bosutinib monohydrate) generic drug versions launch?
Generic name: bosutinib monohydrate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 28, 2026
Generic Entry Controlled by: Canada Patent 2,613,053
BOSULIF is a drug marketed by Pf Prism Cv. There are four patents protecting this drug and two Paragraph IV challenges. Two tentatively approved generics are ready to enter the market.
This drug has eighty-one patent family members in thirty countries. There has been litigation on patents covering BOSULIF
See drug price trends for BOSULIF.
The generic ingredient in BOSULIF is bosutinib monohydrate. There are five drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the bosutinib monohydrate profile page.
When can SIRTURO (bedaquiline fumarate) generic drug versions launch?
Generic name: bedaquiline fumarate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: December 05, 2026
Generic Entry Controlled by: Canada Patent 2,668,512
SIRTURO is a drug marketed by Janssen Therap. There are two patents protecting this drug.
This drug has ninety-seven patent family members in thirty-nine countries.
See drug price trends for SIRTURO.
The generic ingredient in SIRTURO is bedaquiline fumarate. There is one drug master file entry for this API. One supplier is listed for this generic product. Additional details are available on the bedaquiline fumarate profile page.
When can TRADJENTA (linagliptin) generic drug versions launch?
Generic name: linagliptin
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: May 04, 2026
Generic Entry Controlled by: Canada Patent 2,649,922
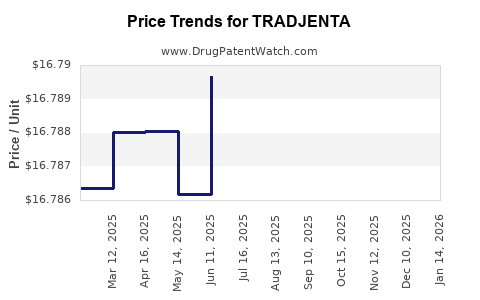
This drug has four hundred and eighty-six patent family members in forty-five countries. There has been litigation on patents covering TRADJENTA
See drug price trends for TRADJENTA.
The generic ingredient in TRADJENTA is linagliptin. There are nineteen drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the linagliptin profile page.
When can XADAGO (safinamide mesylate) generic drug versions launch?
Generic name: safinamide mesylate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 19, 2026
Generic Entry Controlled by: Canada Patent 2,653,012
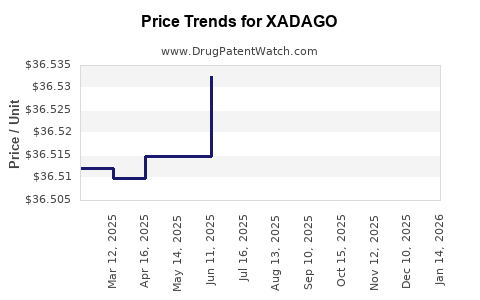
This drug has ninety-seven patent family members in thirty-one countries. There has been litigation on patents covering XADAGO
See drug price trends for XADAGO.
The generic ingredient in XADAGO is safinamide mesylate. There are two drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the safinamide mesylate profile page.
When can CAMCEVI KIT (leuprolide mesylate) generic drug versions launch?
Generic name: leuprolide mesylate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: January 18, 2026
Generic Entry Controlled by: Canada Patent 2,637,569
CAMCEVI KIT is a drug marketed by Accord. There are five patents protecting this drug.
This drug has thirty-nine patent family members in nineteen countries.
The generic ingredient in CAMCEVI KIT is leuprolide mesylate. There are twenty-two drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the leuprolide mesylate profile page.
When can KORSUVA (difelikefalin acetate) generic drug versions launch?
Generic name: difelikefalin acetate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: November 10, 2026
Generic Entry Controlled by: Canada Patent 2,898,514
KORSUVA is a drug marketed by Vifor Intl. There are twelve patents protecting this drug.
This drug has fifty-three patent family members in twenty-seven countries. There has been litigation on patents covering KORSUVA
See drug price trends for KORSUVA.
The generic ingredient in KORSUVA is difelikefalin acetate. One supplier is listed for this generic product. Additional details are available on the difelikefalin acetate profile page.
When can TRIFERIC (ferric pyrophosphate citrate) generic drug versions launch?
Generic name: ferric pyrophosphate citrate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 20, 2026
Generic Entry Controlled by: Canada Patent 2,550,493
TRIFERIC is a drug marketed by Rockwell Medical Inc. There are two patents protecting this drug.
This drug has thirteen patent family members in eleven countries.
See drug price trends for TRIFERIC.
The generic ingredient in TRIFERIC is ferric pyrophosphate citrate. There are twenty drug master file entries for this API. Additional details are available on the ferric pyrophosphate citrate profile page.
When can FARXIGA (dapagliflozin) generic drug versions launch?
Generic name: dapagliflozin
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 28, 2026
Generic Entry Controlled by: Canada Patent 2,985,797
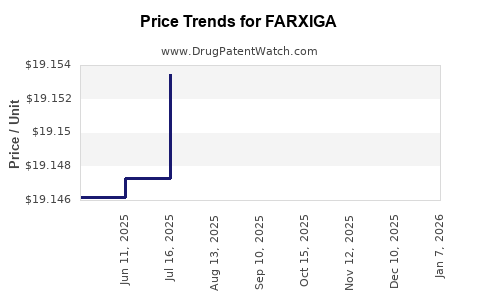
This drug has four hundred and fifty-one patent family members in fifty-two countries. There has been litigation on patents covering FARXIGA
See drug price trends for FARXIGA.
The generic ingredient in FARXIGA is dapagliflozin. There are twenty-six drug master file entries for this API. Five suppliers are listed for this generic product. Additional details are available on the dapagliflozin profile page.
When can QTERN (dapagliflozin; saxagliptin hydrochloride) generic drug versions launch?
Generic name: dapagliflozin; saxagliptin hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 28, 2026
Generic Entry Controlled by: Canada Patent 2,985,797
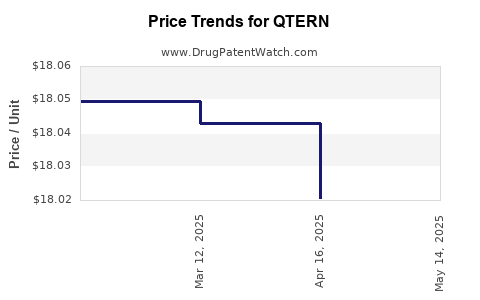
This drug has three hundred and thirteen patent family members in forty-eight countries. There has been litigation on patents covering QTERN
See drug price trends for QTERN.
The generic ingredient in QTERN is dapagliflozin; saxagliptin hydrochloride. There are twenty-six drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the dapagliflozin; saxagliptin hydrochloride profile page.
When can BYDUREON (exenatide synthetic) generic drug versions launch?
Generic name: exenatide synthetic
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 28, 2026
Generic Entry Controlled by: Canada Patent 2,985,797

This drug has three hundred and forty-six patent family members in forty-eight countries. There has been litigation on patents covering BYDUREON
See drug price trends for BYDUREON.
The generic ingredient in BYDUREON is exenatide synthetic. There are seven drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the exenatide synthetic profile page.
When can EPSOLAY (benzoyl peroxide) generic drug versions launch?
Generic name: benzoyl peroxide
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 02, 2026
Generic Entry Controlled by: Canada Patent 2,617,681

This drug has fifty-one patent family members in fifteen countries. There has been litigation on patents covering EPSOLAY
See drug price trends for EPSOLAY.
The generic ingredient in EPSOLAY is benzoyl peroxide. There are seventeen drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the benzoyl peroxide profile page.
When can ZTALMY (ganaxolone) generic drug versions launch?
Generic name: ganaxolone
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: November 28, 2026
Generic Entry Controlled by: Canada Patent 2,631,233
ZTALMY is a drug marketed by Marinus. There are eleven patents protecting this drug.
This drug has forty-eight patent family members in sixteen countries. There has been litigation on patents covering ZTALMY
See drug price trends for ZTALMY.
The generic ingredient in ZTALMY is ganaxolone. One supplier is listed for this generic product. Additional details are available on the ganaxolone profile page.
When can AMELUZ (aminolevulinic acid hydrochloride) generic drug versions launch?
Generic name: aminolevulinic acid hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: December 22, 2026
Generic Entry Controlled by: Canada Patent 2,670,715
AMELUZ is a drug marketed by Biofrontera. There are three patents protecting this drug.
This drug has twenty-nine patent family members in eighteen countries.
See drug price trends for AMELUZ.
The generic ingredient in AMELUZ is aminolevulinic acid hydrochloride. There are six drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the aminolevulinic acid hydrochloride profile page.
When can RAPIVAB (peramivir) generic drug versions launch?
Generic name: peramivir
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 13, 2026
Generic Entry Controlled by: Canada Patent 2,642,260
RAPIVAB is a drug marketed by Biocryst. There are two patents protecting this drug.
This drug has forty-three patent family members in fourteen countries.
See drug price trends for RAPIVAB.
The generic ingredient in RAPIVAB is peramivir. There is one drug master file entry for this API. One supplier is listed for this generic product. Additional details are available on the peramivir profile page.
When can RAPIVAB (peramivir) generic drug versions launch?
Generic name: peramivir
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 13, 2026
Generic Entry Controlled by: Canada Patent 2,649,090
RAPIVAB is a drug marketed by Biocryst. There are two patents protecting this drug.
This drug has forty-three patent family members in fourteen countries.
See drug price trends for RAPIVAB.
The generic ingredient in RAPIVAB is peramivir. There is one drug master file entry for this API. One supplier is listed for this generic product. Additional details are available on the peramivir profile page.
When can ENTRESTO SPRINKLE (sacubitril; valsartan) generic drug versions launch?
Generic name: sacubitril; valsartan
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: November 08, 2026
Generic Entry Controlled by: Canada Patent 2,590,511
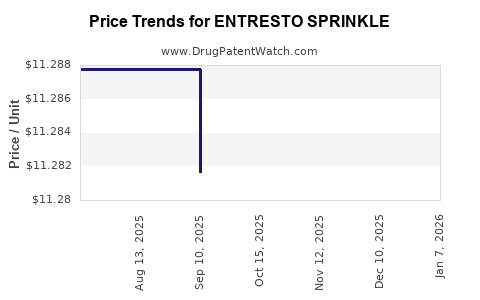
This drug has one hundred and forty-five patent family members in forty-three countries. There has been litigation on patents covering ENTRESTO SPRINKLE
See drug price trends for ENTRESTO SPRINKLE.
The generic ingredient in ENTRESTO SPRINKLE is sacubitril; valsartan. There are eleven drug master file entries for this API. Twenty-two suppliers are listed for this generic product. Additional details are available on the sacubitril; valsartan profile page.
When can ZUNVEYL (benzgalantamine gluconate) generic drug versions launch?
Generic name: benzgalantamine gluconate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: September 22, 2026
Generic Entry Controlled by: Canada Patent 2,623,114
ZUNVEYL is a drug marketed by Alpha Cognition. There are three patents protecting this drug.
This drug has twenty-six patent family members in seventeen countries. There has been litigation on patents covering ZUNVEYL
The generic ingredient in ZUNVEYL is benzgalantamine gluconate. One supplier is listed for this generic product. Additional details are available on the benzgalantamine gluconate profile page.
When can VYKAT XR (diazoxide choline) generic drug versions launch?
Generic name: diazoxide choline
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: January 05, 2026
Generic Entry Controlled by: Canada Patent 2,636,274
VYKAT XR is a drug marketed by Soleno Therap. There are six patents protecting this drug.
This drug has seventy-eight patent family members in twenty-two countries.
The generic ingredient in VYKAT XR is diazoxide choline. There are five drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the diazoxide choline profile page.
When can FINACEA (azelaic acid) generic drug versions launch?
Generic name: azelaic acid
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: January 24, 2026
Generic Entry Controlled by: Canada Patent 2,602,042

This drug has one hundred and thirty-seven patent family members in twenty countries. There has been litigation on patents covering FINACEA
See drug price trends for FINACEA .
The generic ingredient in FINACEA is azelaic acid. There are eight drug master file entries for this API. Nine suppliers are listed for this generic product. Additional details are available on the azelaic acid profile page.
When can FINACEA (azelaic acid) generic drug versions launch?
Generic name: azelaic acid
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: April 18, 2026
Generic Entry Controlled by: Canada Patent 2,606,933

This drug has one hundred and thirty-seven patent family members in twenty countries. There has been litigation on patents covering FINACEA
See drug price trends for FINACEA .
The generic ingredient in FINACEA is azelaic acid. There are eight drug master file entries for this API. Nine suppliers are listed for this generic product. Additional details are available on the azelaic acid profile page.
When can FINACEA (azelaic acid) generic drug versions launch?
Generic name: azelaic acid
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: May 08, 2026
Generic Entry Controlled by: Canada Patent 2,609,948

This drug has one hundred and thirty-seven patent family members in twenty countries. There has been litigation on patents covering FINACEA
See drug price trends for FINACEA .
The generic ingredient in FINACEA is azelaic acid. There are eight drug master file entries for this API. Nine suppliers are listed for this generic product. Additional details are available on the azelaic acid profile page.
When can FINACEA (azelaic acid) generic drug versions launch?
Generic name: azelaic acid
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: May 09, 2026
Generic Entry Controlled by: Canada Patent 2,609,953

This drug has one hundred and thirty-seven patent family members in twenty countries. There has been litigation on patents covering FINACEA
See drug price trends for FINACEA .
The generic ingredient in FINACEA is azelaic acid. There are eight drug master file entries for this API. Nine suppliers are listed for this generic product. Additional details are available on the azelaic acid profile page.
When can FINACEA (azelaic acid) generic drug versions launch?
Generic name: azelaic acid
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: May 09, 2026
Generic Entry Controlled by: Canada Patent 2,610,662

This drug has one hundred and thirty-seven patent family members in twenty countries. There has been litigation on patents covering FINACEA
See drug price trends for FINACEA .
The generic ingredient in FINACEA is azelaic acid. There are eight drug master file entries for this API. Nine suppliers are listed for this generic product. Additional details are available on the azelaic acid profile page.
When can FINACEA (azelaic acid) generic drug versions launch?
Generic name: azelaic acid
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 07, 2026
Generic Entry Controlled by: Canada Patent 2,611,577

This drug has one hundred and thirty-seven patent family members in twenty countries. There has been litigation on patents covering FINACEA
See drug price trends for FINACEA .
The generic ingredient in FINACEA is azelaic acid. There are eight drug master file entries for this API. Nine suppliers are listed for this generic product. Additional details are available on the azelaic acid profile page.
When can NAMZARIC (donepezil hydrochloride; memantine hydrochloride) generic drug versions launch?
Generic name: donepezil hydrochloride; memantine hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: April 06, 2026
Generic Entry Controlled by: Canada Patent 2,604,052
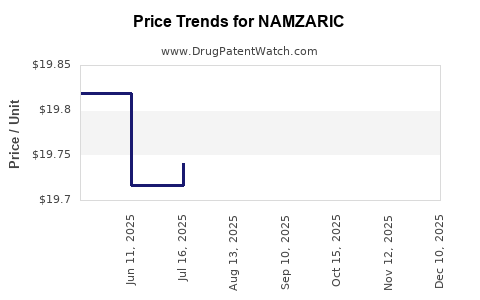
This drug has sixty-seven patent family members in nineteen countries. There has been litigation on patents covering NAMZARIC
See drug price trends for NAMZARIC.
The generic ingredient in NAMZARIC is donepezil hydrochloride; memantine hydrochloride. There are thirty-two drug master file entries for this API. Six suppliers are listed for this generic product. Additional details are available on the donepezil hydrochloride; memantine hydrochloride profile page.
When can REVLIMID (lenalidomide) generic drug versions launch?
Generic name: lenalidomide
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: October 03, 2026
Generic Entry Controlled by: Canada Patent 2,570,755
REVLIMID is a drug marketed by Bristol Myers Squibb. There are two patents protecting this drug and three Paragraph IV challenges.
This drug has three hundred and thirty-one patent family members in forty-one countries. There has been litigation on patents covering REVLIMID
See drug price trends for REVLIMID.
The generic ingredient in REVLIMID is lenalidomide. There are fourteen drug master file entries for this API. Fifteen suppliers are listed for this generic product. Additional details are available on the lenalidomide profile page.
When can REVLIMID (lenalidomide) generic drug versions launch?
Generic name: lenalidomide
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: October 03, 2026
Generic Entry Controlled by: Canada Patent 2,972,299
REVLIMID is a drug marketed by Bristol Myers Squibb. There are two patents protecting this drug and three Paragraph IV challenges.
This drug has three hundred and thirty-one patent family members in forty-one countries. There has been litigation on patents covering REVLIMID
See drug price trends for REVLIMID.
The generic ingredient in REVLIMID is lenalidomide. There are fourteen drug master file entries for this API. Fifteen suppliers are listed for this generic product. Additional details are available on the lenalidomide profile page.
When can VICTRELIS (boceprevir) generic drug versions launch?
Generic name: boceprevir
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: May 31, 2026
Generic Entry Controlled by: Canada Patent 2,611,155
VICTRELIS is a drug marketed by Merck Sharp Dohme. There are two patents protecting this drug.
This drug has twenty-seven patent family members in seventeen countries.
The generic ingredient in VICTRELIS is boceprevir. Additional details are available on the boceprevir profile page.
When can VYVANSE (lisdexamfetamine dimesylate) generic drug versions launch?
Generic name: lisdexamfetamine dimesylate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: April 10, 2026
Generic Entry Controlled by: Canada Patent 2,603,873
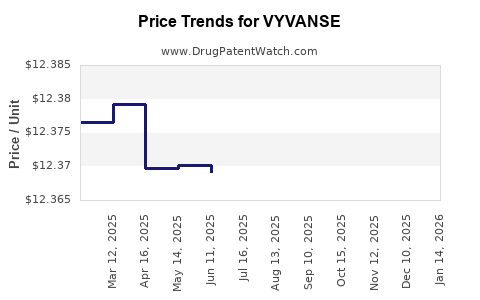
See drug price trends for VYVANSE.
The generic ingredient in VYVANSE is lisdexamfetamine dimesylate. Twenty suppliers are listed for this generic product. Additional details are available on the lisdexamfetamine dimesylate profile page.
When can XTANDI (enzalutamide) generic drug versions launch?
Generic name: enzalutamide
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: March 29, 2026
Generic Entry Controlled by: Canada Patent 2,608,436
XTANDI is a drug marketed by Astellas. There are five patents protecting this drug and one Paragraph IV challenge. Three tentatively approved generics are ready to enter the market.
This drug has one hundred and ninety-one patent family members in thirty-five countries. There has been litigation on patents covering XTANDI
See drug price trends for XTANDI.
The generic ingredient in XTANDI is enzalutamide. There are nine drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the enzalutamide profile page.
When can VALCHLOR (mechlorethamine hydrochloride) generic drug versions launch?
Generic name: mechlorethamine hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: March 14, 2026
Generic Entry Controlled by: Canada Patent 2,600,468
VALCHLOR is a drug marketed by Helsinn. There are six patents protecting this drug.
This drug has fifty patent family members in twenty countries.
See drug price trends for VALCHLOR.
The generic ingredient in VALCHLOR is mechlorethamine hydrochloride. There is one drug master file entry for this API. One supplier is listed for this generic product. Additional details are available on the mechlorethamine hydrochloride profile page.
When can HYLENEX RECOMBINANT (hyaluronidase recombinant human) generic drug versions launch?
Generic name: hyaluronidase recombinant human
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 23, 2026
Generic Entry Controlled by: Canada Patent 2,598,823
HYLENEX RECOMBINANT is a drug marketed by
The generic ingredient in HYLENEX RECOMBINANT is hyaluronidase recombinant human. There are six drug master file entries for this API. Additional details are available on the hyaluronidase recombinant human profile page.
When can EUCRISA (crisaborole) generic drug versions launch?
Generic name: crisaborole
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 16, 2026
Generic Entry Controlled by: Canada Patent 2,597,982
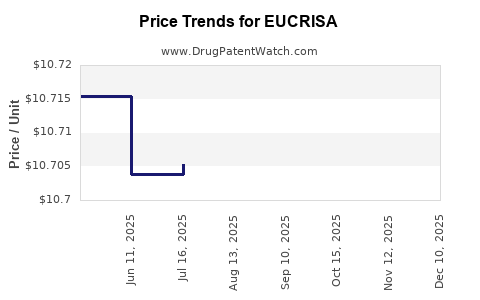
This drug has one hundred and forty-eight patent family members in twenty-eight countries. There has been litigation on patents covering EUCRISA
See drug price trends for EUCRISA.
The generic ingredient in EUCRISA is crisaborole. There are three drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the crisaborole profile page.
When can EUCRISA (crisaborole) generic drug versions launch?
Generic name: crisaborole
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 16, 2026
Generic Entry Controlled by: Canada Patent 2,635,680

This drug has one hundred and forty-eight patent family members in twenty-eight countries. There has been litigation on patents covering EUCRISA
See drug price trends for EUCRISA.
The generic ingredient in EUCRISA is crisaborole. There are three drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the crisaborole profile page.
When can BELEODAQ (belinostat) generic drug versions launch?
Generic name: belinostat
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: May 11, 2026
Generic Entry Controlled by: Canada Patent 2,606,598
BELEODAQ is a drug marketed by Acrotech Biopharma. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has fifty-nine patent family members in twenty-seven countries. There has been litigation on patents covering BELEODAQ
See drug price trends for BELEODAQ.
The generic ingredient in BELEODAQ is belinostat. There are five drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the belinostat profile page.
When can OMONTYS (peginesatide acetate) generic drug versions launch?
Generic name: peginesatide acetate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 05, 2026
Generic Entry Controlled by: Canada Patent 2,609,401
OMONTYS is a drug marketed by Takeda Pharms Usa. There are two patents protecting this drug.
This drug has twenty-seven patent family members in eighteen countries.
The generic ingredient in OMONTYS is peginesatide acetate. Additional details are available on the peginesatide acetate profile page.
When can OTREXUP (methotrexate) generic drug versions launch?
Generic name: methotrexate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: January 24, 2026
Generic Entry Controlled by: Canada Patent 2,595,730
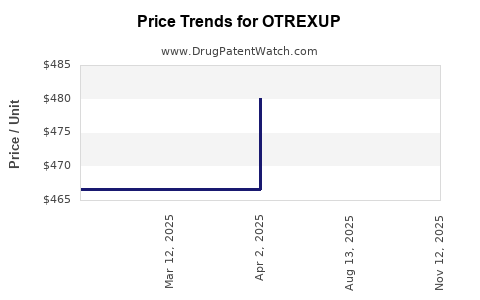
This drug has thirty-nine patent family members in fourteen countries. There has been litigation on patents covering OTREXUP
See drug price trends for OTREXUP.
The generic ingredient in OTREXUP is methotrexate. There are twenty drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the methotrexate profile page.
When can OZEMPIC (semaglutide) generic drug versions launch?
Generic name: semaglutide
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: March 20, 2026
Generic Entry Controlled by: Canada Patent 2,601,784
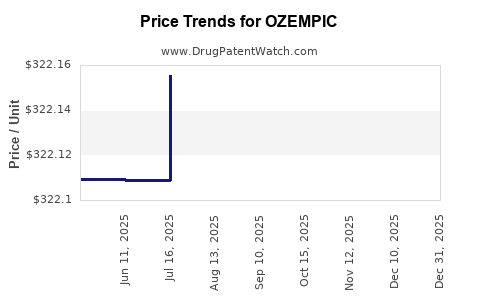
This drug has two hundred and thirty-eight patent family members in thirty-three countries. There has been litigation on patents covering OZEMPIC
See drug price trends for OZEMPIC.
The generic ingredient in OZEMPIC is semaglutide. Two suppliers are listed for this generic product. Additional details are available on the semaglutide profile page.
When can KERYDIN (tavaborole) generic drug versions launch?
Generic name: tavaborole
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 16, 2026
Generic Entry Controlled by: Canada Patent 2,597,982

See drug price trends for KERYDIN.
The generic ingredient in KERYDIN is tavaborole. There are six drug master file entries for this API. Eight suppliers are listed for this generic product. Additional details are available on the tavaborole profile page.
When can KERYDIN (tavaborole) generic drug versions launch?
Generic name: tavaborole
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 16, 2026
Generic Entry Controlled by: Canada Patent 2,635,680

See drug price trends for KERYDIN.
The generic ingredient in KERYDIN is tavaborole. There are six drug master file entries for this API. Eight suppliers are listed for this generic product. Additional details are available on the tavaborole profile page.
When can LO MINASTRIN FE (ethinyl estradiol; norethindrone acetate) generic drug versions launch?
Generic name: ethinyl estradiol; norethindrone acetate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: April 17, 2026
Generic Entry Controlled by: Canada Patent 2,605,299
LO MINASTRIN FE is a drug marketed by Apil. There is one patent protecting this drug. One tentatively approved generic is ready to enter the market.
This drug has nine patent family members in seven countries. There has been litigation on patents covering LO MINASTRIN FE
The generic ingredient in LO MINASTRIN FE is ethinyl estradiol; norethindrone acetate. There are twenty-six drug master file entries for this API. Twenty-six suppliers are listed for this generic product. Additional details are available on the ethinyl estradiol; norethindrone acetate profile page.
When can ACTOPLUS MET XR (metformin hydrochloride; pioglitazone hydrochloride) generic drug versions launch?
Generic name: metformin hydrochloride; pioglitazone hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: March 13, 2026
Generic Entry Controlled by: Canada Patent 2,601,501

This drug has seventy-two patent family members in twenty-five countries. There has been litigation on patents covering ACTOPLUS MET XR
See drug price trends for ACTOPLUS MET XR.
The generic ingredient in ACTOPLUS MET XR is metformin hydrochloride; pioglitazone hydrochloride. There are forty-nine drug master file entries for this API. Seven suppliers are listed for this generic product. Additional details are available on the metformin hydrochloride; pioglitazone hydrochloride profile page.
When can APLENZIN (bupropion hydrobromide) generic drug versions launch?
Generic name: bupropion hydrobromide
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 27, 2026
Generic Entry Controlled by: Canada Patent 2,578,626
APLENZIN is a drug marketed by Bausch. There are eight patents protecting this drug and three Paragraph IV challenges. One tentatively approved generic is ready to enter the market.
This drug has fifty-two patent family members in eighteen countries. There has been litigation on patents covering APLENZIN
See drug price trends for APLENZIN.
The generic ingredient in APLENZIN is bupropion hydrobromide. There are thirty-eight drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the bupropion hydrobromide profile page.
When can TRACLEER (bosentan) generic drug versions launch?
Generic name: bosentan
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: May 15, 2026
Generic Entry Controlled by: Canada Patent 2,607,098
TRACLEER is a drug marketed by Actelion. There are two patents protecting this drug and one Paragraph IV challenge. Two tentatively approved generics are ready to enter the market.
This drug has twenty-eight patent family members in twenty-three countries. There has been litigation on patents covering TRACLEER
See drug price trends for TRACLEER.
The generic ingredient in TRACLEER is bosentan. There are nineteen drug master file entries for this API. Six suppliers are listed for this generic product. Additional details are available on the bosentan profile page.
When can AURYXIA (ferric citrate) generic drug versions launch?
Generic name: ferric citrate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 18, 2026
Generic Entry Controlled by: Canada Patent 2,619,591
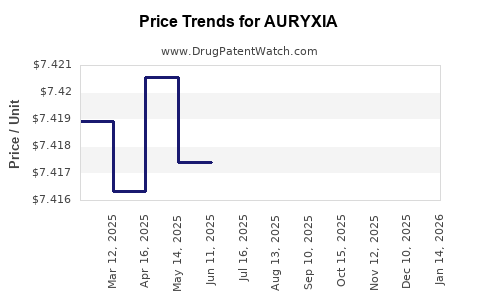
This drug has one hundred and twenty-two patent family members in twenty-three countries. There has been litigation on patents covering AURYXIA
See drug price trends for AURYXIA.
The generic ingredient in AURYXIA is ferric citrate. There are twenty drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the ferric citrate profile page.
When can AURYXIA (ferric citrate) generic drug versions launch?
Generic name: ferric citrate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 18, 2026
Generic Entry Controlled by: Canada Patent 3,050,453

This drug has one hundred and twenty-two patent family members in twenty-three countries. There has been litigation on patents covering AURYXIA
See drug price trends for AURYXIA.
The generic ingredient in AURYXIA is ferric citrate. There are twenty drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the ferric citrate profile page.
When can CORLANOR (ivabradine hydrochloride) generic drug versions launch?
Generic name: ivabradine hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 24, 2026
Generic Entry Controlled by: Canada Patent 2,537,414
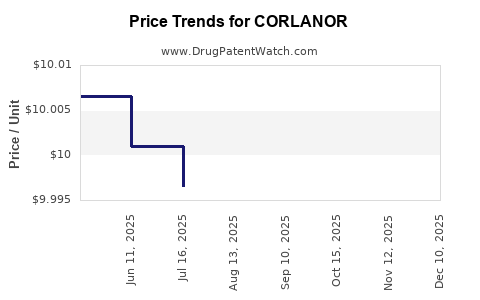
This drug has ninety-seven patent family members in forty-two countries. There has been litigation on patents covering CORLANOR
See drug price trends for CORLANOR.
The generic ingredient in CORLANOR is ivabradine hydrochloride. There are nine drug master file entries for this API. Eleven suppliers are listed for this generic product. Additional details are available on the ivabradine hydrochloride profile page.
When can LATUDA (lurasidone hydrochloride) generic drug versions launch?
Generic name: lurasidone hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: May 26, 2026
Generic Entry Controlled by: Canada Patent 2,606,510
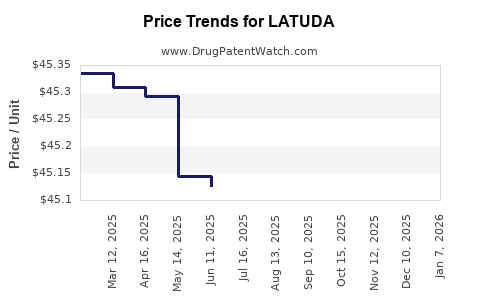
This drug has fifty-eight patent family members in twenty-three countries. There has been litigation on patents covering LATUDA
See drug price trends for LATUDA.
The generic ingredient in LATUDA is lurasidone hydrochloride. There are twenty-six drug master file entries for this API. Thirty suppliers are listed for this generic product. Additional details are available on the lurasidone hydrochloride profile page.
When can LO LOESTRIN FE (ethinyl estradiol; norethindrone acetate) generic drug versions launch?
Generic name: ethinyl estradiol; norethindrone acetate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: April 17, 2026
Generic Entry Controlled by: Canada Patent 2,605,299
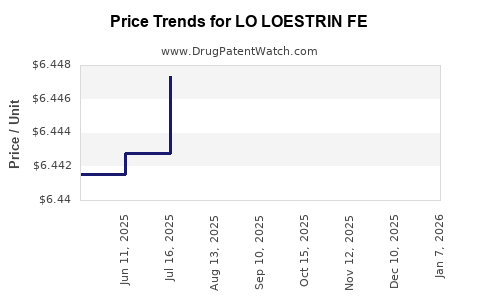
This drug has nine patent family members in seven countries. There has been litigation on patents covering LO LOESTRIN FE
See drug price trends for LO LOESTRIN FE.
The generic ingredient in LO LOESTRIN FE is ethinyl estradiol; norethindrone acetate. There are twenty-six drug master file entries for this API. Twenty-six suppliers are listed for this generic product. Additional details are available on the ethinyl estradiol; norethindrone acetate profile page.
When can NEXAVAR (sorafenib tosylate) generic drug versions launch?
Generic name: sorafenib tosylate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 22, 2026
Generic Entry Controlled by: Canada Patent 2,601,955
NEXAVAR is a drug marketed by Bayer Hlthcare. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has eighty-nine patent family members in thirty-nine countries. There has been litigation on patents covering NEXAVAR
See drug price trends for NEXAVAR.
The generic ingredient in NEXAVAR is sorafenib tosylate. There are thirteen drug master file entries for this API. Eight suppliers are listed for this generic product. Additional details are available on the sorafenib tosylate profile page.
When can TYKERB (lapatinib ditosylate) generic drug versions launch?
Generic name: lapatinib ditosylate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: April 18, 2026
Generic Entry Controlled by: Canada Patent 2,606,207
TYKERB is a drug marketed by Novartis. There is one patent protecting this drug and one Paragraph IV challenge.
This drug has twenty-eight patent family members in twenty-six countries.
See drug price trends for TYKERB.
The generic ingredient in TYKERB is lapatinib ditosylate. There are seven drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the lapatinib ditosylate profile page.
When can XIFAXAN (rifaximin) generic drug versions launch?
Generic name: rifaximin
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 27, 2026
Generic Entry Controlled by: Canada Patent 2,594,789
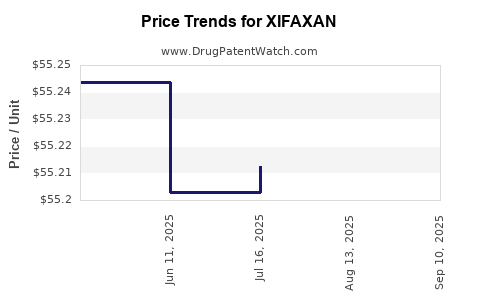
This drug has two hundred and nineteen patent family members in forty-one countries. There has been litigation on patents covering XIFAXAN
See drug price trends for XIFAXAN.
The generic ingredient in XIFAXAN is rifaximin. There are fourteen drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the rifaximin profile page.
When can CORLANOR (ivabradine) generic drug versions launch?
Generic name: ivabradine
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 24, 2026
Generic Entry Controlled by: Canada Patent 2,537,400

This drug has ninety-seven patent family members in forty-two countries. There has been litigation on patents covering CORLANOR
See drug price trends for CORLANOR.
The generic ingredient in CORLANOR is ivabradine. There are nine drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the ivabradine profile page.
When can WAKIX (pitolisant hydrochloride) generic drug versions launch?
Generic name: pitolisant hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 06, 2026
Generic Entry Controlled by: Canada Patent 2,597,016
WAKIX is a drug marketed by Harmony. There are three patents protecting this drug.
This drug has sixty-one patent family members in thirty-one countries. There has been litigation on patents covering WAKIX
See drug price trends for WAKIX.
The generic ingredient in WAKIX is pitolisant hydrochloride. One supplier is listed for this generic product. Additional details are available on the pitolisant hydrochloride profile page.
When can XCOPRI (cenobamate) generic drug versions launch?
Generic name: cenobamate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: April 21, 2026
Generic Entry Controlled by: Canada Patent 2,606,258
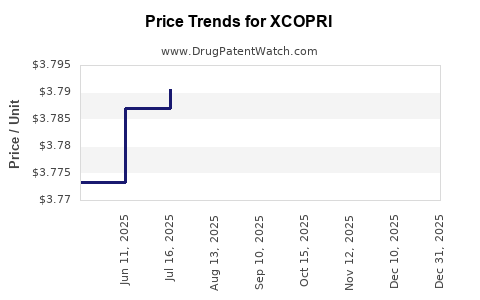
This drug has twenty-six patent family members in twenty countries. There has been litigation on patents covering XCOPRI
See drug price trends for XCOPRI.
The generic ingredient in XCOPRI is cenobamate. One supplier is listed for this generic product. Additional details are available on the cenobamate profile page.
When can JATENZO (testosterone undecanoate) generic drug versions launch?
Generic name: testosterone undecanoate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: April 14, 2026
Generic Entry Controlled by: Canada Patent 2,604,943
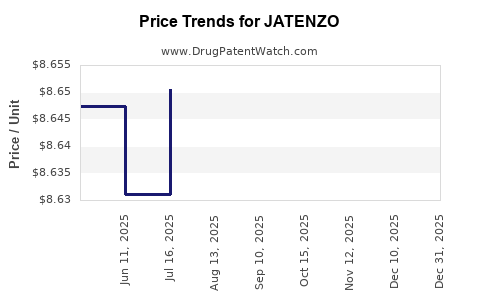
This drug has twenty-nine patent family members in fourteen countries. There has been litigation on patents covering JATENZO
See drug price trends for JATENZO.
The generic ingredient in JATENZO is testosterone undecanoate. There are sixty-nine drug master file entries for this API. Five suppliers are listed for this generic product. Additional details are available on the testosterone undecanoate profile page.
When can BELRAPZO (bendamustine hydrochloride) generic drug versions launch?
Generic name: bendamustine hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: January 13, 2026
Generic Entry Controlled by: Canada Patent 2,593,582
BELRAPZO is a drug marketed by Eagle Pharms. There are eleven patents protecting this drug. Six tentatively approved generics are ready to enter the market.
This drug has sixty-five patent family members in thirty countries. There has been litigation on patents covering BELRAPZO
See drug price trends for BELRAPZO.
The generic ingredient in BELRAPZO is bendamustine hydrochloride. There are twenty-three drug master file entries for this API. Eleven suppliers are listed for this generic product. Additional details are available on the bendamustine hydrochloride profile page.
When can BENDEKA (bendamustine hydrochloride) generic drug versions launch?
Generic name: bendamustine hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: January 13, 2026
Generic Entry Controlled by: Canada Patent 2,593,582
BENDEKA is a drug marketed by Eagle Pharms. There are twenty patents protecting this drug and one Paragraph IV challenge. Six tentatively approved generics are ready to enter the market.
This drug has one hundred and twenty-five patent family members in thirty-one countries. There has been litigation on patents covering BENDEKA
See drug price trends for BENDEKA.
The generic ingredient in BENDEKA is bendamustine hydrochloride. There are twenty-three drug master file entries for this API. Eleven suppliers are listed for this generic product. Additional details are available on the bendamustine hydrochloride profile page.
When can VOCABRIA (cabotegravir sodium) generic drug versions launch?
Generic name: cabotegravir sodium
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: April 28, 2026
Generic Entry Controlled by: Canada Patent 2,606,282
VOCABRIA is a drug marketed by Viiv Hlthcare. There are two patents protecting this drug.
This drug has one hundred and twenty-six patent family members in thirty-three countries.
The generic ingredient in VOCABRIA is cabotegravir sodium. One supplier is listed for this generic product. Additional details are available on the cabotegravir sodium profile page.
When can VOCABRIA (cabotegravir sodium) generic drug versions launch?
Generic name: cabotegravir sodium
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: October 26, 2026
Generic Entry Controlled by: Canada Patent 2,626,956
VOCABRIA is a drug marketed by Viiv Hlthcare. There are two patents protecting this drug.
This drug has one hundred and twenty-six patent family members in thirty-three countries.
The generic ingredient in VOCABRIA is cabotegravir sodium. One supplier is listed for this generic product. Additional details are available on the cabotegravir sodium profile page.
When can WEGOVY (semaglutide) generic drug versions launch?
Generic name: semaglutide
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: March 20, 2026
Generic Entry Controlled by: Canada Patent 2,601,784
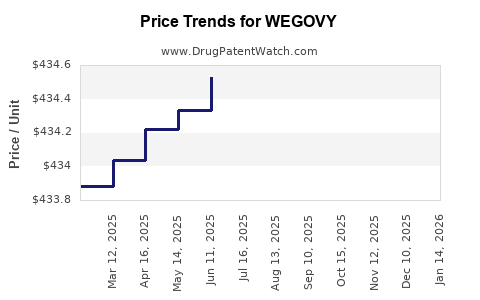
This drug has one hundred and eighty-two patent family members in thirty-three countries. There has been litigation on patents covering WEGOVY
See drug price trends for WEGOVY.
The generic ingredient in WEGOVY is semaglutide. Two suppliers are listed for this generic product. Additional details are available on the semaglutide profile page.
When can REZUROCK (belumosudil mesylate) generic drug versions launch?
Generic name: belumosudil mesylate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: March 27, 2026
Generic Entry Controlled by: Canada Patent 2,602,254
REZUROCK is a drug marketed by Kadmon Pharms Llc. There are five patents protecting this drug.
This drug has fifty-seven patent family members in twenty-nine countries.
See drug price trends for REZUROCK.
The generic ingredient in REZUROCK is belumosudil mesylate. One supplier is listed for this generic product. Additional details are available on the belumosudil mesylate profile page.
When can RECORLEV (levoketoconazole) generic drug versions launch?
Generic name: levoketoconazole
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: January 10, 2026
Generic Entry Controlled by: Canada Patent 2,594,433
RECORLEV is a drug marketed by Strongbridge. There are nine patents protecting this drug.
This drug has thirty-six patent family members in twenty-one countries.
See drug price trends for RECORLEV.
The generic ingredient in RECORLEV is levoketoconazole. One supplier is listed for this generic product. Additional details are available on the levoketoconazole profile page.
When can IZERVAY (avacincaptad pegol sodium) generic drug versions launch?
Generic name: avacincaptad pegol sodium
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 14, 2026
Generic Entry Controlled by: Canada Patent 2,597,889
IZERVAY is a drug marketed by Astellas. There are nine patents protecting this drug.
This drug has one hundred and five patent family members in twenty-nine countries.
See drug price trends for IZERVAY.
The generic ingredient in IZERVAY is avacincaptad pegol sodium. One supplier is listed for this generic product. Additional details are available on the avacincaptad pegol sodium profile page.
When can IZERVAY (avacincaptad pegol sodium) generic drug versions launch?
Generic name: avacincaptad pegol sodium
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 14, 2026
Generic Entry Controlled by: Canada Patent 2,897,900
IZERVAY is a drug marketed by Astellas. There are nine patents protecting this drug.
This drug has one hundred and five patent family members in twenty-nine countries.
See drug price trends for IZERVAY.
The generic ingredient in IZERVAY is avacincaptad pegol sodium. One supplier is listed for this generic product. Additional details are available on the avacincaptad pegol sodium profile page.
When can IZERVAY (avacincaptad pegol sodium) generic drug versions launch?
Generic name: avacincaptad pegol sodium
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 14, 2026
Generic Entry Controlled by: Canada Patent 2,992,874
IZERVAY is a drug marketed by Astellas. There are nine patents protecting this drug.
This drug has one hundred and five patent family members in twenty-nine countries.
See drug price trends for IZERVAY.
The generic ingredient in IZERVAY is avacincaptad pegol sodium. One supplier is listed for this generic product. Additional details are available on the avacincaptad pegol sodium profile page.
When can IZERVAY (avacincaptad pegol sodium) generic drug versions launch?
Generic name: avacincaptad pegol sodium
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 14, 2026
Generic Entry Controlled by: Canada Patent 3,124,030
IZERVAY is a drug marketed by Astellas. There are nine patents protecting this drug.
This drug has one hundred and five patent family members in twenty-nine countries.
See drug price trends for IZERVAY.
The generic ingredient in IZERVAY is avacincaptad pegol sodium. One supplier is listed for this generic product. Additional details are available on the avacincaptad pegol sodium profile page.
DrugPatentWatch cited by CNN, NEJM, Nature Journals, and more …

Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
