KORSUVA Drug Patent Profile
✉ Email this page to a colleague
When do Korsuva patents expire, and what generic alternatives are available?
Korsuva is a drug marketed by Vifor Intl and is included in one NDA. There are twelve patents protecting this drug and one Paragraph IV challenge.
This drug has fifty-three patent family members in twenty-seven countries.
The generic ingredient in KORSUVA is difelikefalin acetate. One supplier is listed for this compound. Additional details are available on the difelikefalin acetate profile page.
DrugPatentWatch® Generic Entry Outlook for Korsuva
Korsuva was eligible for patent challenges on August 23, 2025.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be November 12, 2027. This may change due to patent challenges or generic licensing.
There have been four patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for KORSUVA?
- What are the global sales for KORSUVA?
- What is Average Wholesale Price for KORSUVA?
Summary for KORSUVA
| International Patents: | 53 |
| US Patents: | 12 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 21 |
| Drug Prices: | Drug price information for KORSUVA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for KORSUVA |
| What excipients (inactive ingredients) are in KORSUVA? | KORSUVA excipients list |
| DailyMed Link: | KORSUVA at DailyMed |
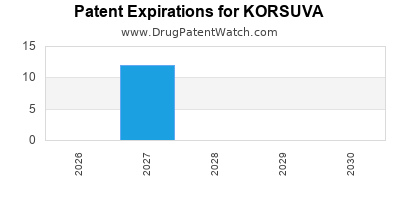

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for KORSUVA
Generic Entry Date for KORSUVA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for KORSUVA
| Drug Class | Kappa Opioid Receptor Agonist |
| Mechanism of Action | Opioid kappa Receptor Agonists |
Paragraph IV (Patent) Challenges for KORSUVA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| KORSUVA | Intravenous Solution | difelikefalin acetate | 0.065 mg/1.3 mL | 214916 | 5 | 2025-08-25 |
US Patents and Regulatory Information for KORSUVA
KORSUVA is protected by twelve US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of KORSUVA is ⤷ Get Started Free.
This potential generic entry date is based on patent 10,017,536.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vifor Intl | KORSUVA | difelikefalin acetate | SOLUTION;INTRAVENOUS | 214916-001 | Aug 23, 2021 | RX | Yes | Yes | 10,793,596 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Vifor Intl | KORSUVA | difelikefalin acetate | SOLUTION;INTRAVENOUS | 214916-001 | Aug 23, 2021 | RX | Yes | Yes | 8,486,894 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Vifor Intl | KORSUVA | difelikefalin acetate | SOLUTION;INTRAVENOUS | 214916-001 | Aug 23, 2021 | RX | Yes | Yes | 9,359,399 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Vifor Intl | KORSUVA | difelikefalin acetate | SOLUTION;INTRAVENOUS | 214916-001 | Aug 23, 2021 | RX | Yes | Yes | 7,402,564 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Vifor Intl | KORSUVA | difelikefalin acetate | SOLUTION;INTRAVENOUS | 214916-001 | Aug 23, 2021 | RX | Yes | Yes | 8,236,766 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Vifor Intl | KORSUVA | difelikefalin acetate | SOLUTION;INTRAVENOUS | 214916-001 | Aug 23, 2021 | RX | Yes | Yes | 10,138,270 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for KORSUVA
When does loss-of-exclusivity occur for KORSUVA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 07317817
Estimated Expiration: ⤷ Get Started Free
Patent: 07319831
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0718651
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 67155
Estimated Expiration: ⤷ Get Started Free
Patent: 67460
Estimated Expiration: ⤷ Get Started Free
Patent: 98514
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1535336
Estimated Expiration: ⤷ Get Started Free
Patent: 1627049
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 16760
Estimated Expiration: ⤷ Get Started Free
Patent: 22032
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 64228
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 64228
Estimated Expiration: ⤷ Get Started Free
Patent: 79756
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 0220043
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1054
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 30814
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 200045
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 7923
Estimated Expiration: ⤷ Get Started Free
Patent: 7924
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 44810
Estimated Expiration: ⤷ Get Started Free
Patent: 64583
Estimated Expiration: ⤷ Get Started Free
Patent: 20180
Estimated Expiration: ⤷ Get Started Free
Patent: 10509343
Estimated Expiration: ⤷ Get Started Free
Patent: 10510966
Estimated Expiration: ⤷ Get Started Free
Patent: 13241447
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 064228
Estimated Expiration: ⤷ Get Started Free
Patent: 2022522
Estimated Expiration: ⤷ Get Started Free
Luxembourg
Patent: 0282
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 8144
Patent: SYNTHETIC PEPTIDE AMIDES
Estimated Expiration: ⤷ Get Started Free
Patent: 3678
Patent: SYNTHETIC PEPTIDE AMIDES AND DIMERS THEREOF
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 09004999
Patent: AMIDAS DE PEPTIDOS SINTETICOS Y DIMEROS DE LAS MISMAS. (SYNTHETIC PEPTIDE AMIDES AND DIMERS THEREOF.)
Estimated Expiration: ⤷ Get Started Free
Patent: 09005000
Patent: AMIDAS DE PEPTIDOS SINTETICOS. (SYNTHETIC PEPTIDE AMIDES.)
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 1199
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 7107
Patent: Synthetic peptide amide ligands of the kappa opiod receptor
Estimated Expiration: ⤷ Get Started Free
Patent: 7108
Patent: Synthetic peptide amides and dimers thereof of the kappa opioid receptor
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 64228
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 64228
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 00685
Patent: СИНТЕТИЧЕСКИЕ ПЕПТИДНЫЕ АМИДЫ (SYNTHETIC PEPTIDE AMIDES)
Estimated Expiration: ⤷ Get Started Free
Patent: 10399
Patent: СИНТЕТИЧЕСКИЕ ПЕПТИДНЫЕ АМИДЫ И ИХ ДИМЕРЫ (SYNTHETIC PEPTIDE AMIDES AND THEIR DIMERS)
Estimated Expiration: ⤷ Get Started Free
Patent: 09121297
Patent: СИНТЕТИЧЕСКИЕ ПЕПТИДНЫЕ АМИДЫ
Estimated Expiration: ⤷ Get Started Free
Patent: 09121298
Patent: СИНТЕТИЧЕСКИЕ ПЕПТИДНЫЕ АМИДЫ И ИХ ДИМЕРЫ
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 64228
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0903053
Patent: SYNTHETIC PEPTIDE AMIDES AND DIMERS THEREOF
Estimated Expiration: ⤷ Get Started Free
Patent: 0903054
Patent: SYNTHETIC PEPTIDE AMIDES
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1513736
Estimated Expiration: ⤷ Get Started Free
Patent: 1513737
Estimated Expiration: ⤷ Get Started Free
Patent: 1513842
Estimated Expiration: ⤷ Get Started Free
Patent: 090085096
Estimated Expiration: ⤷ Get Started Free
Patent: 090085097
Estimated Expiration: ⤷ Get Started Free
Patent: 140056396
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 94377
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering KORSUVA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 101513737 | ⤷ Get Started Free | |
| Israel | 197923 | אמידים פפטידים סינתטיים, תכשירים רוקחיים המכילים אותם ושימושים שלהם (Synthetic peptide amides, pharmaceutical compositions comprising the same and uses thereof) | ⤷ Get Started Free |
| Australia | 2007317817 | ⤷ Get Started Free | |
| Mexico | 2009004999 | AMIDAS DE PEPTIDOS SINTETICOS Y DIMEROS DE LAS MISMAS. (SYNTHETIC PEPTIDE AMIDES AND DIMERS THEREOF.) | ⤷ Get Started Free |
| Russian Federation | 2009121298 | СИНТЕТИЧЕСКИЕ ПЕПТИДНЫЕ АМИДЫ И ИХ ДИМЕРЫ | ⤷ Get Started Free |
| Japan | 2013241447 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for KORSUVA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2064228 | 301199 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: DIFELIKEFALIN, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, HYDRAAT, SOLVAAT, ZUURZOUT-HYDRAAT OF N-OXIDE; REGISTRATION NO/DATE: EU/1/22/1643 20220427 |
| 2064228 | 122022000071 | Germany | ⤷ Get Started Free | PRODUCT NAME: DIFELIKEFALIN IN ALLEN DURCH DAS GRUNDPATENT GESCHUETZTEN FORMEN; REGISTRATION NO/DATE: EU/1/22/1643 20220425 |
| 2064228 | LUC00282 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: DIFELIKEFALIN IN ALL FORMS COVERED BY THE BASIC PATENT; AUTHORISATION NUMBER AND DATE: EU/1/22/1643 20220427 |
| 2064228 | C202230052 | Spain | ⤷ Get Started Free | PRODUCT NAME: DIFELICEFALINA, OPCIONALMENTE EN FORMA DE UNA SAL FARMACEUTICAMENTE ACEPTABLE,HIDRATO, SOLVATO, HIDRATO DE SAL DE ACIDO O N-OXIDO; NATIONAL AUTHORISATION NUMBER: EU/1/22/1643; DATE OF AUTHORISATION: 20220425; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/22/1643; DATE OF FIRST AUTHORISATION IN EEA: 20220425 |
| 2064228 | C02064228/01 | Switzerland | ⤷ Get Started Free | FORMER OWNER: CARA THERAPEUTICS, INC., US |
| 2064228 | 2290040-1 | Sweden | ⤷ Get Started Free | PRODUCT NAME: DIFELIKEFALIN, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, HYDRATE, SOLVATE, OR ACID-SALT-HYDRATE; REG. NO/DATE: EU/1/11/1643 20220427 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for KORSUVA
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
