SIRTURO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Sirturo, and what generic alternatives are available?
Sirturo is a drug marketed by Janssen Therap and is included in one NDA. There are two patents protecting this drug.
This drug has ninety-seven patent family members in thirty-nine countries.
The generic ingredient in SIRTURO is bedaquiline fumarate. There is one drug master file entry for this compound. One supplier is listed for this compound. Additional details are available on the bedaquiline fumarate profile page.
DrugPatentWatch® Generic Entry Outlook for Sirturo
Sirturo was eligible for patent challenges on December 28, 2016.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 19, 2029. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for SIRTURO?
- What are the global sales for SIRTURO?
- What is Average Wholesale Price for SIRTURO?
Summary for SIRTURO
| International Patents: | 97 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 54 |
| Clinical Trials: | 16 |
| Patent Applications: | 156 |
| Drug Prices: | Drug price information for SIRTURO |
| What excipients (inactive ingredients) are in SIRTURO? | SIRTURO excipients list |
| DailyMed Link: | SIRTURO at DailyMed |
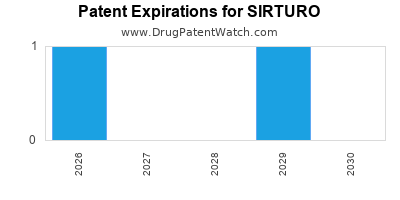

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for SIRTURO
Generic Entry Date for SIRTURO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for SIRTURO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Centers for Disease Control and Prevention | Phase 2/Phase 3 |
| Tuberculosis Trials Consortium | Phase 2/Phase 3 |
| Beijing Chest Hospital | Phase 4 |
Pharmacology for SIRTURO
| Drug Class | Diarylquinoline Antimycobacterial |
US Patents and Regulatory Information for SIRTURO
SIRTURO is protected by two US patents and four FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of SIRTURO is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
International Patents for SIRTURO
When does loss-of-exclusivity occur for SIRTURO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 98
Estimated Expiration: ⤷ Get Started Free
Argentina
Patent: 4149
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 07328945
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0719693
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 68512
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 07003472
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1547904
Estimated Expiration: ⤷ Get Started Free
Patent: 5012303
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0120639
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 13594
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 86940
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 7091
Estimated Expiration: ⤷ Get Started Free
Patent: 0970532
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 86940
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 14513
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 9077
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 94239
Estimated Expiration: ⤷ Get Started Free
Patent: 10511663
Estimated Expiration: ⤷ Get Started Free
Patent: 15028049
Patent: (アルファS,ベータR)−6−ブロモ−アルファ−[2−(ジメチルアミノ)エチル]−2−メトキシ−アルファ−1−ナフタレニル−ベータ−フェニル−3−キノリンエタノールのフマル酸塩 (FUMARATE SALT OF (ALPHA S, BETA R)-6-BROMO-ALPHA-[2-(DIMETHYLAMINO)ETHYL]-2-METHOXY-ALPHA-1-NAPHTHALENYL-BETA-PHENYL-3-QUINOLINEETHANOL)
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 73
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 8844
Patent: FUMARATE SALT OF (ALPHA S, BETA R)-6- BROMO-ALPHA-[2-(DIMETHYLAMINO)ETHYL]-2-METHOXY-ALPHA-1-NAPHTHALENYL-BETA-PHENYL-3-QUINOLINEETHANOL
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 09005909
Patent: SAL FUMARATO DE (ALFA S, BETA R)-6-BROMO-ALFA-[2-(DIMETILAMINO)ETI L]-2-METOXI-ALFA-1-NAFTALENIL-BETA-FENIL-3-QUINOLINAETANOL. (FUMARATE SALT OF (ALPHA S, BETA R)-6-BROMO-ALPHA-[2-(DIMETHYLAMIN O)ETHYL]-2-METHOXY-ALPHA-1-NAPHTHALENYL-BETA-PHENYL-3-QUINOLINEE THANOL.)
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 456
Patent: FUMARATNA SO (ALFA S, BETA R)-6-BROMO-ALFA-[2-(DIMETILAMINO)ETIL]-2-METOKSI-ALFA-1-NAFTALENIL-BETA-FENIL-3-KINOLINETANOLA (FUMARATE SALT OF (ALPHA S, BETA R)-6-BROMO-ALPHA-[2-(DIMETHYLAMINO)ETHYL]-2-METHOXY-ALPHA-1-NAPHTHALENYL-BETA-PHENYL-3-QUINOLINEETHANOL)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 6485
Patent: FUMARATE SALT OF (ALPHA S, BETA R)-6-BROMO-ALPHA-[2-(DIMETHYLAMINO)ETHYL]-2-METHOXY-ALPHA-1-NAPHTHALENYL-BETA-PHENYL-3-QUINOLINEETHANOL
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 2773
Estimated Expiration: ⤷ Get Started Free
Patent: 092535
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 081350
Patent: SAL FUMARATO DE (ALFAS S, BETA R)-6-BROMO-ALFA-[2(DIMETILAMINO)ETIL]-2-METOXI-ALFA-1-NAFTALENIL-BETA-FENIL-3-QUINOLINAETANOL
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 86940
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 86940
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 408
Patent: FUMARATNA SO (ALFA S, BETA R)-6-BROMO-ALFA-[2-(DIMETILAMINO)ETIL]-2-METOKSI-ALFA-1-NAFTALENIL-BETA-FENIL-3-KINOLINETANOLA (FUMARATE SALT OF (ALPHA S, BETA R)-6-BROMO-ALPHA-[2-(DIMETHYLAMINO)ETHYL]-2¬METHOXY-ALPHA-1-NAPHTHALENYL-BETA-PHENYL-3-QUlNOLINEETHANOL)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 86940
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0903907
Patent: FUMARATE SALT OF (ALPHA S,BETA R)-6-BROMO-ALPHA-[2-(DIMETHYLAMINO)ETHYL]-2-METHOXY-ALPHA-1-NAPHTHALENYL-BETA-PHENYL-3-QUINOLINEETHANOL
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1514700
Estimated Expiration: ⤷ Get Started Free
Patent: 090087020
Patent: FUMARATE SALT OF (ALPHA S, BETA R)-6-BROMO-ALPHA-[2-(DIMETHYLAMINO)ETHYL]-2-METHOXY-ALPHA-1-NAPHTHALENYL-BETA-PHENYL-3-QUINOLINEETHANOL
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 87923
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 17098
Estimated Expiration: ⤷ Get Started Free
Patent: 0838527
Patent: Fumarate salt of (alpha S, beta R)-6-bromo-alpha-[2-(dimethylamino)ethyl]-2-methoxy-alpha-1-naphthalenyl-beta-phenyl-3-quinolineethanol
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 813
Patent: ФУМАРАТНАЯ СОЛЬ (АЛЬФА S, БЕТА R)-6-БРОМ-АЛЬФА-[2-(ДИМЕТИЛАМИНО)ЭТИЛ]-2-МЕТОКСИ-АЛЬФА-1-НАФТАЛЕНИЛ-БЕТА-ФЕНИЛ-3-ХИНОЛИНЭТАНОЛА;ФУМАРАТНА СІЛЬ (АЛЬФА S, БЕТА R)-6-БРОМ-АЛЬФА-[2-(ДИМЕТИЛАМІНО)ЕТИЛ]-2-МЕТОКСІ-АЛЬФА-1-НАФТАЛЕНІЛ-БЕТА-ФЕНІЛ-3-ХІНОЛІНЕТАНОЛУ (FUMARATE SALT OF (ALPHA S, BETA R)-6-BROMO-ALPHA-[2-(DIMETHYLAMINO)ETHYL]-2-METHOXY-ALPHA-1-NAPHTHALENYL-BETA-PHENYL-3-QUINOLINEETHANOL)
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 762
Patent: SAL FUMARATO DE (ALFA S, BETA R)-6-BROMO-ALFA-[2(DIMETILAMINO) ETIL]-2-METOXI-ALFA-1-NAFTALENIL-BETA-FENIL-3-QUINOLINAETANOL
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering SIRTURO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Eurasian Patent Organization | 200500257 | ПРОИЗВОДНЫЕ ХИНОЛИНА И ИХ ПРИМЕНЕНИЕ В КАЧЕСТВЕ МИКОБАКТЕРИАЛЬНЫХ ИНГИБИТОРОВ | ⤷ Get Started Free |
| Eurasian Patent Organization | 008937 | ПРОИЗВОДНЫЕ ХИНОЛИНА И ИХ ПРИМЕНЕНИЕ В КАЧЕСТВЕ МИКОБАКТЕРИАЛЬНЫХ ИНГИБИТОРОВ (QUINOLINE DERIVATIVES AND THEIR USE AS MYCOBACTERIAL INHIBITORS) | ⤷ Get Started Free |
| South Korea | 20050033607 | QUINOLINE DERIVATIVES AND THEIR USE AS MYCOBACTERIAL INHIBITORS | ⤷ Get Started Free |
| Jordan | 2973 | ⤷ Get Started Free | |
| Denmark | 2301544 | ⤷ Get Started Free | |
| Iceland | 2914 | ⤷ Get Started Free | |
| Denmark | 1527050 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for SIRTURO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1527050 | SPC/GB14/057 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: BEDAQUILINE, OR A PHARMACEUTICALLY ACCEPTABLE ACID OR BASE ADDITION SALT THEREOF, INCLUDING BEDAQUILINE FUMARATE; REGISTERED: UK EU/1/13/901 20140307 |
| 1527050 | 14C0060 | France | ⤷ Get Started Free | PRODUCT NAME: BEDAQUILINE OU UN DE SES SELS D'ADDITION D'ACIDE OU DE BASE PHARMACEUTIQUEMENT ACCEPTABLE, Y COMPRIS LE FUMARATE DE BEDAQUILINE; REGISTRATION NO/DATE: EU/1/13/901 20140307 |
| 1527050 | PA2014032,C1527050 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: BEDAQUILINUM; REGISTRATION NO/DATE: EU/1/13/901 20140305 |
| 1527050 | C20140026 00112 | Estonia | ⤷ Get Started Free | PRODUCT NAME: BEDAKVILIIN;REG NO/DATE: K(2014)1616 (LOPLIK) 07.03.2014 |
| 1527050 | 300684 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: BEDAQUILINE, OF EEN FARMACEUTISCH AANVAARDBAAR ZUUR- OF BASE-ADDITIEZOUT DAARVAN, WAARONDER BEDAQUILINEFUMARAAT; REGISTRATION NO/DATE: EU/1/13/901 20140307 |
| 1527050 | C300684 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: BEDAQUILINE, OF EEN FARMACEUTISCH AANVAARDBAAR ZUUR- OF BASE-ADDITIEZOUT DAARVAN, WAARONDER BEDAQUILINEFUMARAAT; REGISTRATION NO/DATE: EU/1/13/901 20140307 |
| 1527050 | 122014000083 | Germany | ⤷ Get Started Free | PRODUCT NAME: BEDAQUILIN ODER EIN PHARMAZEUTISCH ANNEHMBARES SAEURE- ODER BASENADDITIONSSALZ DAVON; REGISTRATION NO/DATE: EU/1/13/901 20140305 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for the Pharmaceutical Drug: SIRTURO
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
