KYBELLA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Kybella, and when can generic versions of Kybella launch?
Kybella is a drug marketed by Abbvie and is included in one NDA. There are sixteen patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and forty-seven patent family members in thirty-eight countries.
The generic ingredient in KYBELLA is deoxycholic acid. There are thirty-three drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the deoxycholic acid profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Kybella
A generic version of KYBELLA was approved as deoxycholic acid by WILSHIRE PHARMS INC on April 2nd, 2021.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for KYBELLA?
- What are the global sales for KYBELLA?
- What is Average Wholesale Price for KYBELLA?
Summary for KYBELLA
| International Patents: | 147 |
| US Patents: | 16 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 98 |
| Clinical Trials: | 17 |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for KYBELLA |
| What excipients (inactive ingredients) are in KYBELLA? | KYBELLA excipients list |
| DailyMed Link: | KYBELLA at DailyMed |
Recent Clinical Trials for KYBELLA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| T-TOP Clinical Research Co., Ltd. | PHASE1 |
| Glonova Pharma Co., Ltd | PHASE1 |
| AbbVie | PHASE4 |
Pharmacology for KYBELLA
| Drug Class | Cytolytic Agent |
| Physiological Effect | Decreased Cell Membrane Integrity |
Paragraph IV (Patent) Challenges for KYBELLA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| KYBELLA | Injection | deoxycholic acid | 10 mg/mL (2 mL) | 206333 | 1 | 2018-07-13 |
US Patents and Regulatory Information for KYBELLA
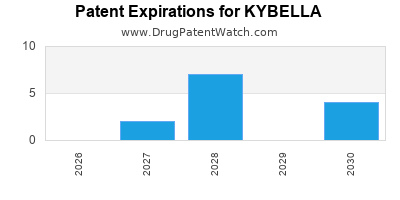
KYBELLA is protected by sixteen US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbvie | KYBELLA | deoxycholic acid | SOLUTION;SUBCUTANEOUS | 206333-001 | Apr 29, 2015 | AP | RX | Yes | Yes | 7,754,230 | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Abbvie | KYBELLA | deoxycholic acid | SOLUTION;SUBCUTANEOUS | 206333-001 | Apr 29, 2015 | AP | RX | Yes | Yes | 9,636,349 | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Abbvie | KYBELLA | deoxycholic acid | SOLUTION;SUBCUTANEOUS | 206333-001 | Apr 29, 2015 | AP | RX | Yes | Yes | 7,622,130 | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Abbvie | KYBELLA | deoxycholic acid | SOLUTION;SUBCUTANEOUS | 206333-001 | Apr 29, 2015 | AP | RX | Yes | Yes | 8,367,649 | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for KYBELLA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | KYBELLA | deoxycholic acid | SOLUTION;SUBCUTANEOUS | 206333-001 | Apr 29, 2015 | 8,298,556 | ⤷ Get Started Free |
| Abbvie | KYBELLA | deoxycholic acid | SOLUTION;SUBCUTANEOUS | 206333-001 | Apr 29, 2015 | 8,846,066 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for KYBELLA
When does loss-of-exclusivity occur for KYBELLA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 7440
Patent: ÁCIDO BILIAR SINTETICO, UNA COMPOSICION QUE LO COMPRENDE, UN METODO PARA SU PREPARACION, COMPUESTOS INTERMEDIARIOS DE SíNTESIS Y SU EMPLEO EN LA ELIMINACION DE LOS DEPoSITOS DE GRASA CORPORAL
Estimated Expiration: ⤷ Get Started Free
Patent: 2325
Patent: MÉTODO DE PREPARACIÓN DEL ÁCIDO BILIAR SINTÉTICO
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 08265721
Patent: Synthetic bile acid composition, method, and preparation
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0813140
Patent: COMPOSIÇÃO, MÉTODO E PREPARAÇÃO DE ÁCIDO BILIAR SINTÉTICO
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 90841
Patent: COMPOSITION, PROCEDE ET PREPARATION D'ACIDE BILIAIRE SYNTHETIQUE (SYNTHETIC BILE ACID COMPOSITION, METHOD, AND PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 89109
Patent: COMPOSITION, PROCEDE ET PREPARATION D'ACIDE BILIAIRE SYNTHETIQUE (SYNTHETIC BILE ACID COMPOSITION, METHOD, AND PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 08001783
Patent: Acido desoxicolico (dca); composicion farmaceutica que lo comprende; procedimiento de preparacion de acido desoxicolico; compuestos intermediarios, utiles en la preparacion de un medicamento para remover depositos de grasa.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1711254
Patent: Synthetic bile acid composition, method, and preparation
Estimated Expiration: ⤷ Get Started Free
Patent: 6083969
Patent: 合成胆汁酸组合物、方法和制剂 (Synthetic bile acid composition, method, and preparation)
Estimated Expiration: ⤷ Get Started Free
Patent: 6146594
Patent: 合成胆汁酸组合物、方法和制剂 (Synthetic bile acid composition, method, and preparation)
Estimated Expiration: ⤷ Get Started Free
Patent: 8191940
Patent: 合成胆汁酸组合物、方法和制剂 (Synthetic bile acid composition, method, and preparation)
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 174
Patent: COMPOSICION, METODO Y PREPARACION DEL ACIDO BILIAR SINTETICO
Estimated Expiration: ⤷ Get Started Free
Patent: 170070
Patent: COMPOSICIÓN, MÉTODO Y PREPARACIÓN DEL ÁCIDO BILIAR SINTÉTICO
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0150879
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 16700
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 07475
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 0806
Patent: СПОСОБ ПОЛУЧЕНИЯ ДЕЗОКСИХОЛЕВОЙ КИСЛОТЫ (ВАРИАНТЫ), ДЕЗОКСИХОЛЕВАЯ КИСЛОТА, ПОЛУЧЕННАЯ УКАЗАННЫМ СПОСОБОМ, ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ, ЕЕ СОДЕРЖАЩАЯ, СПОСОБ УДАЛЕНИЯ ЖИРОВЫХ ОТЛОЖЕНИЙ (METHOD FOR PRERARING DEOXYCHOLIC ACID (EMBODIMENTS), DEOXYCHOLIC ACID PREPARED BY SAID METHOD, PHARMACEUTICAL COMPOSITION CONTAINING SAME, METHOD FOR REMOVAL OF FAT DEPOSITS)
Estimated Expiration: ⤷ Get Started Free
Patent: 1000032
Patent: СИНТЕТИЧЕСКАЯ ДЕЗОКСИХОЛЕВАЯ КИСЛОТА И КОМПОЗИЦИЯ НА ЕЕ ОСНОВЕ, СОЕДИНЕНИЯ И СПОСОБ ПОЛУЧЕНИЯ ДЕЗОКСИХОЛЕВОЙ КИСЛОТЫ, СПОСОБ УДАЛЕНИЯ ЖИРОВЫХ ОТЛОЖЕНИЙ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 69383
Patent: COMPOSITION, PROCÉDÉ ET PRÉPARATION D'ACIDE BILIAIRE SYNTHÉTIQUE (SYNTHETIC BILE ACID COMPOSITION, METHOD, AND PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 07475
Patent: préparation synthétique d'acide biliaire (synthetic bile acid preparation)
Estimated Expiration: ⤷ Get Started Free
Patent: 02290
Patent: SYNTHESE D'ACIDE DESOXYCHOLIQUE (DCA) (SYNTHESIS OF DEOXYCHOLIC ACID (DCA))
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 65807
Patent: 合成的胆汁酸製備方法 (SYNTHETIC BILE ACID PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 23372
Patent: 脫氧膽酸 的合成 (SYNTHESIS OF DEOXYCHOLIC ACID (DCA) (DCA))
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 25909
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 2203
Patent: תכשיר חומצת מרה מלאכותי ושיטה להכנת החומצה (Synthetic bile acid composition and method for preparation of the bile acid)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 89102
Estimated Expiration: ⤷ Get Started Free
Patent: 10530876
Estimated Expiration: ⤷ Get Started Free
Patent: 13075918
Patent: SYNTHETIC BILE ACID COMPOSITION, METHOD THEREFOR AND PREPARATION THEREOF
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 0180077
Patent: تركيبات وطرق لحمض صفراوي تخليقي (SYNTHETIC BILE ACID COMPOSITIONS AND METHODS)
Estimated Expiration: ⤷ Get Started Free
Patent: 72
Patent: تركيبة وطريقة وتحضير الصفراء الصناعية (SYNTHETIC BILE ACID COMPOSITION,METHOD,AND PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 0040
Patent: SYNTHETIC BILE ACID COMPOSITION, METHOD AND PREPARATION
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 0094
Patent: COMPOSICIÓN, MÉTODO Y PREPARACIÓN DEL ÁCIDO BILIAR SINTÉTICO. (SYNTHETIC BILE ACID COMPOSITION, METHOD, AND PREPARATION.)
Estimated Expiration: ⤷ Get Started Free
Patent: 09013664
Patent: COMPOSICION, METODO Y PREPARACION DEL ACIDO BILIAR SINTETICO. (SYNTHETIC BILE ACID COMPOSITION, METHOD, AND PREPARATION.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 1081
Patent: SYNTHETIC DEOXYCHOLIC ACID (DCA) OR AN ESTER, HYDROXAMALE, OR HYDROXYAMIDE THEREOF
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 013500364
Patent: SYNTHETIC BILE ACID COMPOSITION, METHOD, AND PREPARATION
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 07475
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 07475
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 201000003
Patent: Composizione, metodo e preparazione di acidi biliari sintetici
Estimated Expiration: ⤷ Get Started Free
Patent: 01000003
Patent: Composizione, metodo e preparazione di acidi biliari sintetici.
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 07475
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0907928
Patent: SYNETHOC BILE ACID COMPOSITION, METHOD, AND PREPARATION
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1598402
Estimated Expiration: ⤷ Get Started Free
Patent: 1863131
Estimated Expiration: ⤷ Get Started Free
Patent: 1916024
Estimated Expiration: ⤷ Get Started Free
Patent: 2061001
Estimated Expiration: ⤷ Get Started Free
Patent: 100031512
Patent: SYNTHETIC BILE ACID COMPOSITION, METHOD, AND PREPARATION
Estimated Expiration: ⤷ Get Started Free
Patent: 130133881
Patent: SYNTHETIC BILE ACID COMPOSITION, METHOD, AND PREPARATION
Estimated Expiration: ⤷ Get Started Free
Patent: 150013305
Patent: SYNTHETIC BILE ACID COMPOSITION, METHOD, AND PREPARATION
Estimated Expiration: ⤷ Get Started Free
Patent: 160025044
Patent: 합성 담즙산 조성물, 방법 및 제조물 (SYNTHETIC BILE ACID COMPOSITION, METHOD, AND PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 160150646
Patent: 합성 담즙산 조성물, 방법 및 제조물 (SYNTHETIC BILE ACID COMPOSITION METHOD AND PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 180061401
Patent: 합성 담즙산 조성물, 방법 및 제조물 (SYNTHETIC BILE ACID COMPOSITION METHOD AND PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 180122483
Patent: 합성 담즙산 조성물, 방법 및 제조물 (SYNTHETIC BILE ACID COMPOSITION METHOD AND PREPARATION)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 50504
Estimated Expiration: ⤷ Get Started Free
Patent: 26429
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 03327
Estimated Expiration: ⤷ Get Started Free
Patent: 03396
Estimated Expiration: ⤷ Get Started Free
Patent: 0918550
Patent: Synthetic bile acid composition, method, and preparation
Estimated Expiration: ⤷ Get Started Free
Patent: 1534684
Patent: Synthetic bile acid composition, method, and preparation
Estimated Expiration: ⤷ Get Started Free
United Kingdom
Patent: 07615
Estimated Expiration: ⤷ Get Started Free
Patent: 12493
Estimated Expiration: ⤷ Get Started Free
Patent: 52358
Patent: Preparation of bile acids and intermediates thereof
Estimated Expiration: ⤷ Get Started Free
Patent: 60350
Patent: Preparation of bile acids and intermediates thereof
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering KYBELLA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Cyprus | 1120146 | ⤷ Get Started Free | |
| South Korea | 20160150646 | 합성 담즙산 조성물, 방법 및 제조물 (SYNTHETIC BILE ACID COMPOSITION METHOD AND PREPARATION) | ⤷ Get Started Free |
| Cyprus | 1112204 | ⤷ Get Started Free | |
| South Korea | 101916024 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for KYBELLA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2380576 | 41/2020 | Austria | ⤷ Get Started Free | PRODUCT NAME: DESOXYCHOLSAEURE-NATRIUMSALZ; NAT. REGISTRATION NO/DATE: 137169 20160825; FIRST REGISTRATION: IS IS/1/16/071/01 20160729 |
| 2380576 | SPC/GB20/050 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: DEOXYCHOLIC ACID SODIUM SALT; REGISTERED: UK PL 45496/0009 20170526 |
| 1758590 | LUC00029 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: SEL DE SODIUM D'ACIDE DEOXYCHOLIQUE; AUTHORISATION NUMBER AND DATE: IS/1/16/071/01 20170401 |
| 2380576 | 2020/043 | Ireland | ⤷ Get Started Free | PRODUCT NAME: DEOXYCHOLIC ACID SODIUM SALT; NAT REGISTRATION NO/DATE: PA2103/003/001 20170602; FIRST REGISTRATION NO/DATE: SE/H/1547/001/DC 20160729 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for KYBELLA: A Comprehensive Analysis
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
