TRADJENTA Drug Patent Profile
✉ Email this page to a colleague
When do Tradjenta patents expire, and what generic alternatives are available?
Tradjenta is a drug marketed by Boehringer Ingelheim and is included in one NDA. There are eleven patents protecting this drug and one Paragraph IV challenge.
This drug has four hundred and eighty-six patent family members in forty-five countries.
The generic ingredient in TRADJENTA is linagliptin. There are nineteen drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the linagliptin profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Tradjenta
A generic version of TRADJENTA was approved as linagliptin by SUNSHINE on August 31st, 2021.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TRADJENTA?
- What are the global sales for TRADJENTA?
- What is Average Wholesale Price for TRADJENTA?
Summary for TRADJENTA
| International Patents: | 486 |
| US Patents: | 11 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 93 |
| Clinical Trials: | 10 |
| Drug Prices: | Drug price information for TRADJENTA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TRADJENTA |
| What excipients (inactive ingredients) are in TRADJENTA? | TRADJENTA excipients list |
| DailyMed Link: | TRADJENTA at DailyMed |
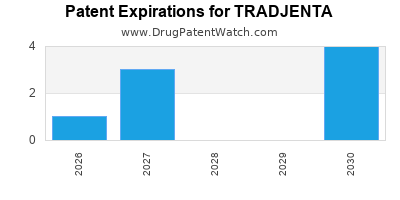
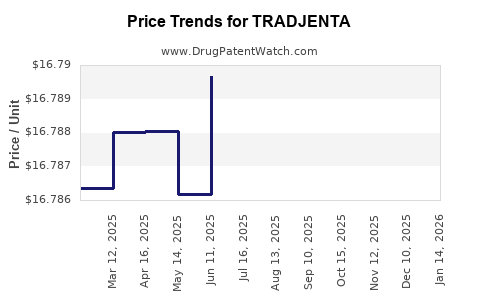
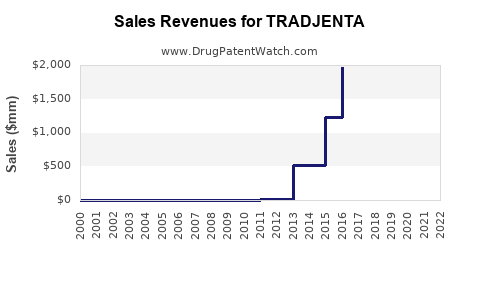
Recent Clinical Trials for TRADJENTA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| EMS | Phase 3 |
| University of Miami | Phase 4 |
| Northwell Health | Phase 4 |
Pharmacology for TRADJENTA
| Drug Class | Dipeptidyl Peptidase 4 Inhibitor |
| Mechanism of Action | Dipeptidyl Peptidase 4 Inhibitors |
Paragraph IV (Patent) Challenges for TRADJENTA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| TRADJENTA | Tablets | linagliptin | 5 mg | 201280 | 11 | 2015-05-04 |
US Patents and Regulatory Information for TRADJENTA
TRADJENTA is protected by eleven US patents and two FDA Regulatory Exclusivities.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | AB | RX | Yes | Yes | 11,911,388 | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | AB | RX | Yes | Yes | 8,853,156*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | AB | RX | Yes | Yes | 8,846,695*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | AB | RX | Yes | Yes | 10,034,877*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TRADJENTA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | 6,303,661 | ⤷ Get Started Free |
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | 8,119,648 | ⤷ Get Started Free |
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | 7,459,428 | ⤷ Get Started Free |
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | 6,890,898 | ⤷ Get Started Free |
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | 7,078,381 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for TRADJENTA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim International GmbH | Trajenta | linagliptin | EMEA/H/C/002110Trajenta is indicated in the treatment of type 2 diabetes mellitus to improve glycaemic control in adults:as monotherapyin patients inadequately controlled by diet and exercise alone and for whom metformin is inappropriate due to intolerance, or contraindicated due to renal impairment.as combination therapyin combination with metformin when diet and exercise plus metformin alone do not provide adequate glycaemic control.in combination with a sulphonylurea and metformin when diet and exercise plus dual therapy with these medicinal products do not provide adequate glycaemic control.in combination with insulin with or without metformin, when this regimen alone, with diet and exercise, does not provide adequate glycaemic control. | Authorised | no | no | no | 2011-08-23 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for TRADJENTA
When does loss-of-exclusivity occur for TRADJENTA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 0755
Estimated Expiration: ⤷ Get Started Free
Patent: 9930
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 07247193
Estimated Expiration: ⤷ Get Started Free
Austria
Patent: 80228
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0711179
Estimated Expiration: ⤷ Get Started Free
Patent: 0722388
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 49922
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 12002521
Estimated Expiration: ⤷ Get Started Free
Patent: 12002522
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1437493
Estimated Expiration: ⤷ Get Started Free
Patent: 2526737
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0100507
Estimated Expiration: ⤷ Get Started Free
Patent: 0150003
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 11354
Estimated Expiration: ⤷ Get Started Free
Patent: 16064
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 23902
Estimated Expiration: ⤷ Get Started Free
Patent: 77509
Estimated Expiration: ⤷ Get Started Free
Patent: 83819
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 088800
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 6559
Estimated Expiration: ⤷ Get Started Free
Patent: 9890
Estimated Expiration: ⤷ Get Started Free
Patent: 0802184
Estimated Expiration: ⤷ Get Started Free
Patent: 1100958
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 52108
Estimated Expiration: ⤷ Get Started Free
Patent: 23902
Estimated Expiration: ⤷ Get Started Free
Patent: 77509
Estimated Expiration: ⤷ Get Started Free
Patent: 83819
Estimated Expiration: ⤷ Get Started Free
Patent: 10241
Estimated Expiration: ⤷ Get Started Free
Germany
Patent: 2007009091
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 30442
Estimated Expiration: ⤷ Get Started Free
Patent: 72549
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 25210
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 5030
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 78244
Estimated Expiration: ⤷ Get Started Free
Patent: 00998
Estimated Expiration: ⤷ Get Started Free
Patent: 64720
Estimated Expiration: ⤷ Get Started Free
Patent: 87908
Estimated Expiration: ⤷ Get Started Free
Patent: 84711
Estimated Expiration: ⤷ Get Started Free
Patent: 09535376
Estimated Expiration: ⤷ Get Started Free
Patent: 12072187
Estimated Expiration: ⤷ Get Started Free
Patent: 13227338
Estimated Expiration: ⤷ Get Started Free
Patent: 16104811
Estimated Expiration: ⤷ Get Started Free
Patent: 18021082
Estimated Expiration: ⤷ Get Started Free
Patent: 20079316
Estimated Expiration: ⤷ Get Started Free
Patent: 22075826
Estimated Expiration: ⤷ Get Started Free
Patent: 24074800
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 6969
Estimated Expiration: ⤷ Get Started Free
Patent: 8496
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 8617
Estimated Expiration: ⤷ Get Started Free
Patent: 4206
Estimated Expiration: ⤷ Get Started Free
Patent: 08013958
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 170
Estimated Expiration: ⤷ Get Started Free
Patent: 941
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 2862
Estimated Expiration: ⤷ Get Started Free
Patent: 5983
Estimated Expiration: ⤷ Get Started Free
Patent: 3426
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 3067
Estimated Expiration: ⤷ Get Started Free
Patent: 084256
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 080698
Patent: COMPOSICIONES FARMACEUTICAS DE INHIBIDORES DE DPP IV
Estimated Expiration: ⤷ Get Started Free
Patent: 110666
Patent: COMPOSICIONES FARMACEUTICAS DE INHIBIDORES DE DPP IV
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 23902
Estimated Expiration: ⤷ Get Started Free
Patent: 77509
Estimated Expiration: ⤷ Get Started Free
Patent: 83819
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 23902
Estimated Expiration: ⤷ Get Started Free
Patent: 83819
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 466
Patent: FORMULACIJE DPP IV INHIBITORA (DPP IV INHIBITOR FORMULATIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 570
Patent: FORMULACIJE DPP IV INHIBITORA (DPP IV INHIBITOR FORMULATIONS)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 1649
Patent: DPP IV INHIBITOR FORMULATIONS
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 23902
Estimated Expiration: ⤷ Get Started Free
Patent: 83819
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0808361
Patent: DPP IV inhibitor formulations
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1478983
Estimated Expiration: ⤷ Get Started Free
Patent: 1710881
Estimated Expiration: ⤷ Get Started Free
Patent: 1855323
Estimated Expiration: ⤷ Get Started Free
Patent: 2051281
Estimated Expiration: ⤷ Get Started Free
Patent: 090009226
Estimated Expiration: ⤷ Get Started Free
Patent: 140063896
Estimated Expiration: ⤷ Get Started Free
Patent: 150100957
Estimated Expiration: ⤷ Get Started Free
Patent: 160128446
Estimated Expiration: ⤷ Get Started Free
Patent: 170141812
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 48576
Estimated Expiration: ⤷ Get Started Free
Patent: 27409
Estimated Expiration: ⤷ Get Started Free
Patent: 38818
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 74843
Estimated Expiration: ⤷ Get Started Free
Patent: 20753
Estimated Expiration: ⤷ Get Started Free
Patent: 0812648
Patent: DPP IV inhibitor formulations
Estimated Expiration: ⤷ Get Started Free
Patent: 1417844
Patent: DPP IV inhibitor formulations
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 942
Patent: ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИИ С ИНГИБИТОРАМИ DPP IV;ФАРМАЦЕВТИЧНІ КОМПОЗИЦІЇ З ІНГІБІТОРАМИ DPP IV (DPP IV INHIBITOR FORMULATIONS)
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 319
Patent: FORMULACIONES DE INHIBIDORES DE DPP IV
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TRADJENTA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2397142 | ⤷ Get Started Free | |
| China | 1675212 | 8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions | ⤷ Get Started Free |
| Brazil | PI0920699 | ⤷ Get Started Free | |
| Chile | 2012002528 | ⤷ Get Started Free | |
| European Patent Office | 1084705 | Méthode pour diminuer le taux de glucose sanguin des mammifères (Method for lowering blood glucose levels in mammals) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TRADJENTA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0896538 | 07C0035 | France | ⤷ Get Started Free | PRODUCT NAME: SITAGLIPTIN; REGISTRATION NO/DATE IN FRANCE: EU/1/07/383/001 DU 20070321; REGISTRATION NO/DATE AT EEC: EU/1/07/383/001-018 DU 20070321 |
| 0896538 | 300308 | Netherlands | ⤷ Get Started Free | 300308, 20170424, EXPIRES: 20220423 |
| 1084705 | SPC/GB14/085 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: SAXAGLIPTIN; REGISTERED: UK EU/1/09/545/001-010 20091005 |
| 0896538 | 91382 | Luxembourg | ⤷ Get Started Free | 91382, EXPIRES: 20220424 |
| 1084705 | C01084705/04 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: LINAGLIPTIN; REGISTRATION NO/DATE: SWISSMEDIC 61893 08.03.2012 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for the Pharmaceutical Drug: TRADJENTA
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
