47 Drugs Facing NCE-1 / Abbreviated New Drug Application acceptance dates in 2024 - 2025
Loss of Exclusivity / End of Market Exclusivity Period dates
The content of this page is licensed under a Creative Commons Attribution 4.0 International License.

Friedman, Yali, "47 Drugs Facing NCE-1 / Abbreviated New Drug Application acceptance dates in 2024 - 2025" DrugPatentWatch.com thinkBiotech, 2024 www.drugpatentwatch.com/p/nce-1/.
Media collateral
These NCE-1 dates indicate the first opportunity for generic drug companies to file Abbreviated New Drug Applications (ANDAs) for generic entry into branded drug markets. Generic launch is dependent on many factors, including FDA approval and patents. This information is provided as a rough estimate of generic application, and does not indicate when generics will launch. For more information see the complete DrugPatentWatch database.
When can drug patent challenges be filed against VEKLURY?
Generic name: remdesivir
NCE-1 Date: October 2024
VEKLURY is a drug marketed by Gilead Sciences Inc. There are sixteen patents protecting this drug.
This drug has three hundred and twenty-five patent family members in forty-seven countries.
The generic ingredient in VEKLURY is remdesivir. Additional details are available on the remdesivir profile page.
When can drug patent challenges be filed against OLINVYK?
Generic name: oliceridine
NCE-1 Date: October 2024
OLINVYK is a drug marketed by Trevena. There are five patents protecting this drug.
This drug has fifty-one patent family members in twenty-three countries.
See drug price trends for OLINVYK.
The generic ingredient in OLINVYK is oliceridine. Additional details are available on the oliceridine profile page.
When can drug patent challenges be filed against ZOKINVY?
Generic name: lonafarnib
NCE-1 Date: November 2024
ZOKINVY is a drug marketed by Sentynl Theraps Inc. There are two patents protecting this drug.
This drug has nine patent family members in six countries.
See drug price trends for ZOKINVY.
The generic ingredient in ZOKINVY is lonafarnib. Additional details are available on the lonafarnib profile page.
When can drug patent challenges be filed against OXLUMO?
Generic name: lumasiran sodium
NCE-1 Date: November 2024
OXLUMO is a drug marketed by Alnylam Pharms Inc. There are fourteen patents protecting this drug.
This drug has one hundred and seventy-one patent family members in forty-two countries.
See drug price trends for OXLUMO.
The generic ingredient in OXLUMO is lumasiran sodium. Additional details are available on the lumasiran sodium profile page.
When can drug patent challenges be filed against IMCIVREE?
Generic name: setmelanotide acetate
NCE-1 Date: November 2024
IMCIVREE is a drug marketed by Rhythm. There are three patents protecting this drug.
This drug has ninety-four patent family members in twenty-one countries.
See drug price trends for IMCIVREE.
The generic ingredient in IMCIVREE is setmelanotide acetate. Additional details are available on the setmelanotide acetate profile page.
When can drug patent challenges be filed against GALLIUM GA 68 GOZETOTIDE?
Generic name: gallium ga-68 gozetotide
NCE-1 Date: December 2024
GALLIUM GA 68 GOZETOTIDE is a drug marketed by Univ Ca Los Angeles and Univ Of Ca San Fran
This drug has ninety-four patent family members in twenty-one countries.
The generic ingredient in GALLIUM GA 68 GOZETOTIDE is gallium ga-68 gozetotide. There are sixteen drug master file entries for this API. Additional details are available on the gallium ga-68 gozetotide profile page.
When can drug patent challenges be filed against ORLADEYO?
Generic name: berotralstat hydrochloride
NCE-1 Date: December 2024
ORLADEYO is a drug marketed by Biocryst. There are eight patents protecting this drug.
This drug has seventy-nine patent family members in thirty-four countries.
See drug price trends for ORLADEYO.
The generic ingredient in ORLADEYO is berotralstat hydrochloride. Additional details are available on the berotralstat hydrochloride profile page.
When can drug patent challenges be filed against KLISYRI?
Generic name: tirbanibulin
NCE-1 Date: December 2024
KLISYRI is a drug marketed by Almirall. There are eight patents protecting this drug.
This drug has one hundred and eight patent family members in twenty-six countries.
See drug price trends for KLISYRI.
The generic ingredient in KLISYRI is tirbanibulin. Additional details are available on the tirbanibulin profile page.
When can drug patent challenges be filed against MYFEMBREE?
Generic name: estradiol; norethindrone acetate; relugolix
NCE-1 Date: December 2024
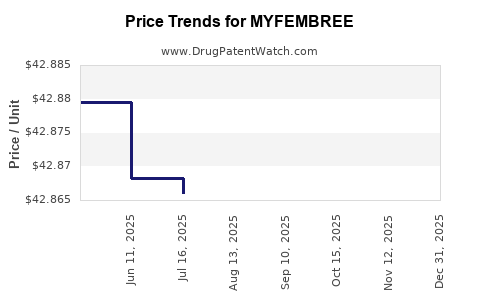
This drug has one hundred and thirty-four patent family members in thirty-four countries.
See drug price trends for MYFEMBREE.
The generic ingredient in MYFEMBREE is estradiol; norethindrone acetate; relugolix. There are seventy-five drug master file entries for this API. Additional details are available on the estradiol; norethindrone acetate; relugolix profile page.
When can drug patent challenges be filed against ORGOVYX?
Generic name: relugolix
NCE-1 Date: December 2024
ORGOVYX is a drug marketed by Sumitomo Pharma. There are eight patents protecting this drug.
This drug has one hundred and sixty-two patent family members in thirty-six countries.
See drug price trends for ORGOVYX.
The generic ingredient in ORGOVYX is relugolix. Additional details are available on the relugolix profile page.
When can drug patent challenges be filed against GEMTESA?
Generic name: vibegron
NCE-1 Date: December 2024
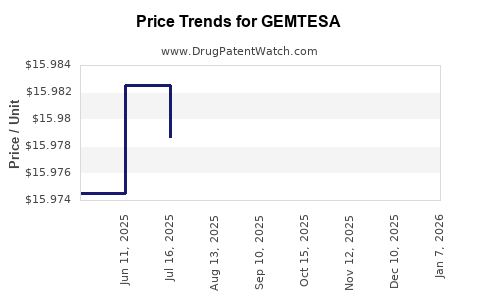
This drug has fifty-five patent family members in forty countries.
See drug price trends for GEMTESA.
The generic ingredient in GEMTESA is vibegron. Additional details are available on the vibegron profile page.
When can drug patent challenges be filed against VERQUVO?
Generic name: vericiguat
NCE-1 Date: January 2025
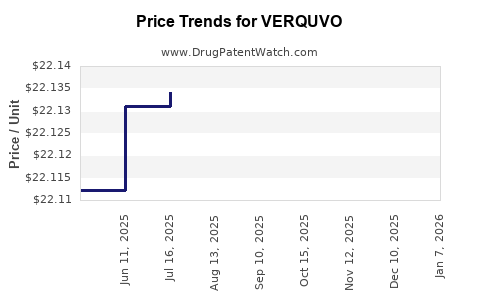
This drug has two hundred and ten patent family members in forty-nine countries.
See drug price trends for VERQUVO.
The generic ingredient in VERQUVO is vericiguat. Additional details are available on the vericiguat profile page.
When can drug patent challenges be filed against APRETUDE?
Generic name: cabotegravir
NCE-1 Date: January 2025
APRETUDE is a drug marketed by Viiv Hlthcare. There are three patents protecting this drug.
This drug has one hundred and fifty-seven patent family members in thirty-eight countries.
See drug price trends for APRETUDE.
The generic ingredient in APRETUDE is cabotegravir. Additional details are available on the cabotegravir profile page.
When can drug patent challenges be filed against CABENUVA KIT?
Generic name: cabotegravir; rilpivirine
NCE-1 Date: January 2025
CABENUVA KIT is a drug marketed by Viiv Hlthcare. There are five patents protecting this drug.
This drug has three hundred and ninety-two patent family members in fifty-one countries. There has been litigation on patents covering CABENUVA KIT
The generic ingredient in CABENUVA KIT is cabotegravir; rilpivirine. Additional details are available on the cabotegravir; rilpivirine profile page.
When can drug patent challenges be filed against VOCABRIA?
Generic name: cabotegravir sodium
NCE-1 Date: January 2025
VOCABRIA is a drug marketed by Viiv Hlthcare. There are two patents protecting this drug.
This drug has one hundred and twenty-five patent family members in thirty-three countries.
The generic ingredient in VOCABRIA is cabotegravir sodium. Additional details are available on the cabotegravir sodium profile page.
When can drug patent challenges be filed against LUPKYNIS?
Generic name: voclosporin
NCE-1 Date: January 2025
LUPKYNIS is a drug marketed by Aurinia. There are three patents protecting this drug.
This drug has one hundred and ninety-three patent family members in thirty-nine countries.
See drug price trends for LUPKYNIS.
The generic ingredient in LUPKYNIS is voclosporin. There is one drug master file entry for this API. Additional details are available on the voclosporin profile page.
When can drug patent challenges be filed against TEPMETKO?
Generic name: tepotinib hydrochloride
NCE-1 Date: February 2025
TEPMETKO is a drug marketed by Emd Serono Inc. There are eight patents protecting this drug.
This drug has seventy-six patent family members in thirty-five countries.
See drug price trends for TEPMETKO.
The generic ingredient in TEPMETKO is tepotinib hydrochloride. Additional details are available on the tepotinib hydrochloride profile page.
When can drug patent challenges be filed against UKONIQ?
Generic name: umbralisib tosylate
NCE-1 Date: February 2025
UKONIQ is a drug marketed by Tg Theraps. There are eight patents protecting this drug.
This drug has sixty-eight patent family members in thirty-one countries.
The generic ingredient in UKONIQ is umbralisib tosylate. Additional details are available on the umbralisib tosylate profile page.
When can drug patent challenges be filed against COSELA?
Generic name: trilaciclib dihydrochloride
NCE-1 Date: February 2025
COSELA is a drug marketed by G1 Therap. There are twelve patents protecting this drug.
This drug has one hundred and thirty-five patent family members in twenty-seven countries.
See drug price trends for COSELA.
The generic ingredient in COSELA is trilaciclib dihydrochloride. Additional details are available on the trilaciclib dihydrochloride profile page.
When can drug patent challenges be filed against AMONDYS 45?
Generic name: casimersen
NCE-1 Date: February 2025
AMONDYS 45 is a drug marketed by Sarepta Theraps Inc. There are six patents protecting this drug.
This drug has eighty-seven patent family members in twenty-four countries.
The generic ingredient in AMONDYS 45 is casimersen. Additional details are available on the casimersen profile page.
When can drug patent challenges be filed against NULIBRY?
Generic name: fosdenopterin hydrobromide
NCE-1 Date: February 2025
NULIBRY is a drug marketed by Sentynl Theraps Inc. There is one patent protecting this drug.
This drug has ten patent family members in ten countries.
See drug price trends for NULIBRY.
The generic ingredient in NULIBRY is fosdenopterin hydrobromide. Additional details are available on the fosdenopterin hydrobromide profile page.
When can drug patent challenges be filed against FOTIVDA?
Generic name: tivozanib hydrochloride
NCE-1 Date: March 2025
FOTIVDA is a drug marketed by Aveo Pharms. There are three patents protecting this drug.
This drug has fifty-five patent family members in twenty-six countries.
See drug price trends for FOTIVDA.
The generic ingredient in FOTIVDA is tivozanib hydrochloride. Additional details are available on the tivozanib hydrochloride profile page.
When can drug patent challenges be filed against PONVORY?
Generic name: ponesimod
NCE-1 Date: March 2025
PONVORY is a drug marketed by Vanda Pharms Inc. There are six patents protecting this drug.
This drug has one hundred and forty-four patent family members in forty countries.
See drug price trends for PONVORY.
The generic ingredient in PONVORY is ponesimod. Additional details are available on the ponesimod profile page.
When can drug patent challenges be filed against ZEGALOGUE?
Generic name: dasiglucagon hydrochloride
NCE-1 Date: March 2025
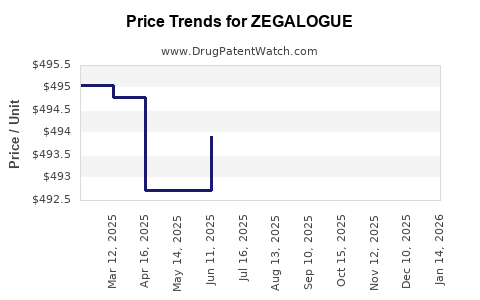
This drug has thirty-three patent family members in twenty-four countries.
See drug price trends for ZEGALOGUE.
The generic ingredient in ZEGALOGUE is dasiglucagon hydrochloride. Additional details are available on the dasiglucagon hydrochloride profile page.
When can drug patent challenges be filed against ZEGALOGUE (AUTOINJECTOR)?
Generic name: dasiglucagon hydrochloride
NCE-1 Date: March 2025
ZEGALOGUE (AUTOINJECTOR) is a drug marketed by Zealand Pharma. There are two patents protecting this drug.
This drug has thirty-three patent family members in twenty-four countries.
The generic ingredient in ZEGALOGUE (AUTOINJECTOR) is dasiglucagon hydrochloride. Additional details are available on the dasiglucagon hydrochloride profile page.
When can drug patent challenges be filed against QELBREE?
Generic name: viloxazine hydrochloride
NCE-1 Date: April 2025
QELBREE is a drug marketed by Supernus Pharms. There are five patents protecting this drug.
This drug has twenty-one patent family members in seven countries.
See drug price trends for QELBREE.
The generic ingredient in QELBREE is viloxazine hydrochloride. Additional details are available on the viloxazine hydrochloride profile page.
When can drug patent challenges be filed against NEXTSTELLIS?
Generic name: drospirenone; estetrol
NCE-1 Date: April 2025
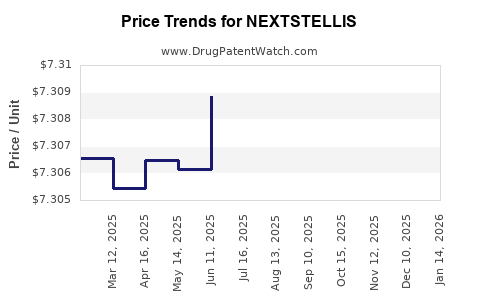
This drug has two hundred and two patent family members in forty-seven countries.
See drug price trends for NEXTSTELLIS.
The generic ingredient in NEXTSTELLIS is drospirenone; estetrol. There are eleven drug master file entries for this API. Additional details are available on the drospirenone; estetrol profile page.
When can drug patent challenges be filed against AZSTARYS?
Generic name: dexmethylphenidate hydrochloride; serdexmethylphenidate chloride
NCE-1 Date: May 2025
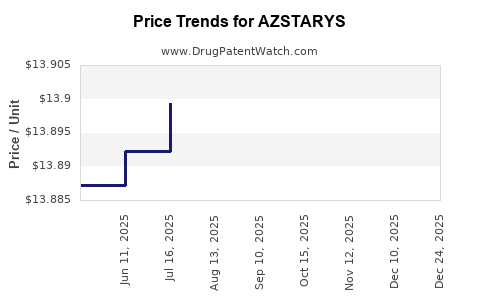
This drug has eighty-one patent family members in twenty-nine countries.
See drug price trends for AZSTARYS.
The generic ingredient in AZSTARYS is dexmethylphenidate hydrochloride; serdexmethylphenidate chloride. There are six drug master file entries for this API. Additional details are available on the dexmethylphenidate hydrochloride; serdexmethylphenidate chloride profile page.
When can drug patent challenges be filed against SYFOVRE?
Generic name: pegcetacoplan
NCE-1 Date: May 2025
SYFOVRE is a drug marketed by Apellis Pharms. There are eleven patents protecting this drug.
This drug has one hundred and eighty patent family members in twenty-six countries.
See drug price trends for SYFOVRE.
The generic ingredient in SYFOVRE is pegcetacoplan. Additional details are available on the pegcetacoplan profile page.
When can drug patent challenges be filed against EMPAVELI?
Generic name: pegcetacoplan
NCE-1 Date: May 2025
EMPAVELI is a drug marketed by Apellis Pharms. There are ten patents protecting this drug.
This drug has one hundred and forty-five patent family members in twenty-six countries.
See drug price trends for EMPAVELI.
The generic ingredient in EMPAVELI is pegcetacoplan. Additional details are available on the pegcetacoplan profile page.
When can drug patent challenges be filed against PYLARIFY?
Generic name: piflufolastat f-18
NCE-1 Date: May 2025
PYLARIFY is a drug marketed by Progenics Pharms Inc. There are five patents protecting this drug.
This drug has ninety-six patent family members in twenty-four countries.
See drug price trends for PYLARIFY.
The generic ingredient in PYLARIFY is piflufolastat f-18. Additional details are available on the piflufolastat f-18 profile page.
When can drug patent challenges be filed against LUMAKRAS?
Generic name: sotorasib
NCE-1 Date: May 2025
LUMAKRAS is a drug marketed by Amgen Inc. There are four patents protecting this drug.
This drug has one hundred and eighteen patent family members in thirty-six countries.
See drug price trends for LUMAKRAS.
The generic ingredient in LUMAKRAS is sotorasib. Additional details are available on the sotorasib profile page.
When can drug patent challenges be filed against LYBALVI?
Generic name: olanzapine; samidorphan l-malate
NCE-1 Date: May 2025
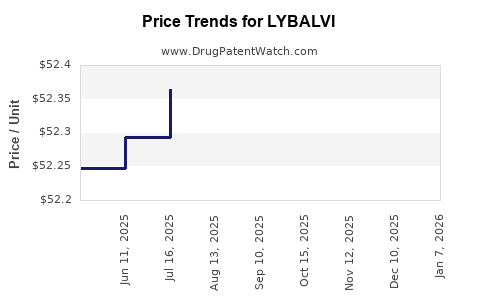
This drug has eighty-five patent family members in twenty-seven countries.
See drug price trends for LYBALVI.
The generic ingredient in LYBALVI is olanzapine; samidorphan l-malate. There are thirty-three drug master file entries for this API. Additional details are available on the olanzapine; samidorphan l-malate profile page.
When can drug patent challenges be filed against TRUSELTIQ?
Generic name: infigratinib phosphate
NCE-1 Date: May 2025
TRUSELTIQ is a drug marketed by Helsinn Hlthcare. There are four patents protecting this drug.
This drug has one hundred and thirty-five patent family members in forty countries.
See drug price trends for TRUSELTIQ.
The generic ingredient in TRUSELTIQ is infigratinib phosphate. Additional details are available on the infigratinib phosphate profile page.
When can drug patent challenges be filed against BREXAFEMME?
Generic name: ibrexafungerp citrate
NCE-1 Date: June 2025

This drug has fifty patent family members in twenty-four countries.
See drug price trends for BREXAFEMME.
The generic ingredient in BREXAFEMME is ibrexafungerp citrate. Additional details are available on the ibrexafungerp citrate profile page.
When can drug patent challenges be filed against KERENDIA?
Generic name: finerenone
NCE-1 Date: July 2025
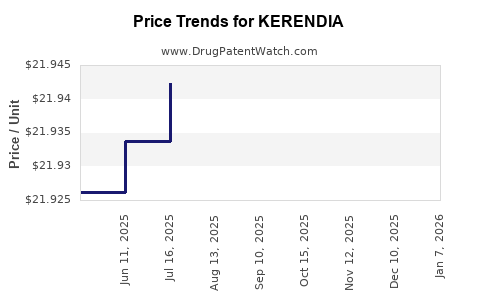
This drug has ninety-three patent family members in forty-seven countries.
See drug price trends for KERENDIA.
The generic ingredient in KERENDIA is finerenone. Additional details are available on the finerenone profile page.
When can drug patent challenges be filed against FEXINIDAZOLE?
Generic name: fexinidazole
NCE-1 Date: July 2025
FEXINIDAZOLE is a drug marketed by Sanofi.
This drug has ninety-three patent family members in forty-seven countries.
The generic ingredient in FEXINIDAZOLE is fexinidazole. Additional details are available on the fexinidazole profile page.
When can drug patent challenges be filed against REZUROCK?
Generic name: belumosudil mesylate
NCE-1 Date: July 2025
REZUROCK is a drug marketed by Kadmon Pharms Llc. There are five patents protecting this drug.
This drug has forty-seven patent family members in twenty-seven countries.
See drug price trends for REZUROCK.
The generic ingredient in REZUROCK is belumosudil mesylate. Additional details are available on the belumosudil mesylate profile page.
When can drug patent challenges be filed against BYLVAY?
Generic name: odevixibat
NCE-1 Date: July 2025
BYLVAY is a drug marketed by Ipsen. There are eleven patents protecting this drug.
This drug has one hundred and twelve patent family members in forty countries.
See drug price trends for BYLVAY.
The generic ingredient in BYLVAY is odevixibat. Additional details are available on the odevixibat profile page.
When can drug patent challenges be filed against WELIREG?
Generic name: belzutifan
NCE-1 Date: August 2025
WELIREG is a drug marketed by Merck Sharp Dohme. There are two patents protecting this drug.
This drug has fifty-three patent family members in twenty-eight countries.
See drug price trends for WELIREG.
The generic ingredient in WELIREG is belzutifan. Additional details are available on the belzutifan profile page.
When can drug patent challenges be filed against KORSUVA?
Generic name: difelikefalin acetate
NCE-1 Date: August 2025
KORSUVA is a drug marketed by Cara Therap. There are twelve patents protecting this drug.
This drug has fifty patent family members in twenty-five countries.
See drug price trends for KORSUVA.
The generic ingredient in KORSUVA is difelikefalin acetate. Additional details are available on the difelikefalin acetate profile page.
When can drug patent challenges be filed against EXKIVITY?
Generic name: mobocertinib succinate
NCE-1 Date: September 2025
EXKIVITY is a drug marketed by Takeda Pharms Usa. There are two patents protecting this drug.
This drug has sixty-two patent family members in thirty-nine countries.
See drug price trends for EXKIVITY.
The generic ingredient in EXKIVITY is mobocertinib succinate. Additional details are available on the mobocertinib succinate profile page.
When can drug patent challenges be filed against QULIPTA?
Generic name: atogepant
NCE-1 Date: September 2025
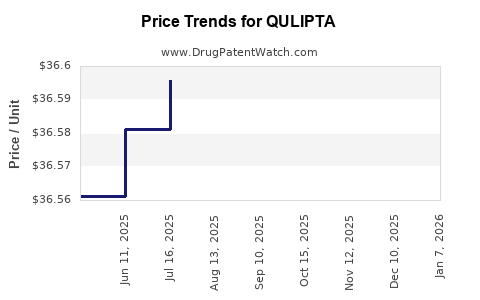
This drug has one hundred and two patent family members in forty-five countries. There has been litigation on patents covering QULIPTA
See drug price trends for QULIPTA.
The generic ingredient in QULIPTA is atogepant. Additional details are available on the atogepant profile page.
When can drug patent challenges be filed against LIVMARLI?
Generic name: maralixibat chloride
NCE-1 Date: September 2025
LIVMARLI is a drug marketed by Mirum. There are seven patents protecting this drug.
This drug has one hundred and fourteen patent family members in twenty-two countries.
See drug price trends for LIVMARLI.
The generic ingredient in LIVMARLI is maralixibat chloride. Additional details are available on the maralixibat chloride profile page.
When can drug patent challenges be filed against TAVNEOS?
Generic name: avacopan
NCE-1 Date: October 2025
TAVNEOS is a drug marketed by Chemocentryx. There are four patents protecting this drug.
This drug has one hundred and eight patent family members in thirty-seven countries.
See drug price trends for TAVNEOS.
The generic ingredient in TAVNEOS is avacopan. Additional details are available on the avacopan profile page.
When can drug patent challenges be filed against SCEMBLIX?
Generic name: asciminib hydrochloride
NCE-1 Date: October 2025
SCEMBLIX is a drug marketed by Novartis. There are two patents protecting this drug.
This drug has eighty-four patent family members in forty-nine countries.
See drug price trends for SCEMBLIX.
The generic ingredient in SCEMBLIX is asciminib hydrochloride. Additional details are available on the asciminib hydrochloride profile page.
When can drug patent challenges be filed against VOXZOGO?
Generic name: vosoritide
NCE-1 Date: November 2025
VOXZOGO is a drug marketed by Biomarin Pharm. There are six patents protecting this drug.
This drug has eighty patent family members in twenty-six countries.
See drug price trends for VOXZOGO.
The generic ingredient in VOXZOGO is vosoritide. Additional details are available on the vosoritide profile page.



