KAZANO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Kazano, and what generic alternatives are available?
Kazano is a drug marketed by Takeda Pharms Usa and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has ninety patent family members in forty-one countries.
The generic ingredient in KAZANO is alogliptin benzoate; metformin hydrochloride. There are ten drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the alogliptin benzoate; metformin hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Kazano
Kazano was eligible for patent challenges on January 25, 2017.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be May 24, 2029. This may change due to patent challenges or generic licensing.
There have been five patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for KAZANO?
- What are the global sales for KAZANO?
- What is Average Wholesale Price for KAZANO?
Summary for KAZANO
| International Patents: | 90 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 1 |
| Patent Applications: | 1 |
| Drug Prices: | Drug price information for KAZANO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for KAZANO |
| What excipients (inactive ingredients) are in KAZANO? | KAZANO excipients list |
| DailyMed Link: | KAZANO at DailyMed |
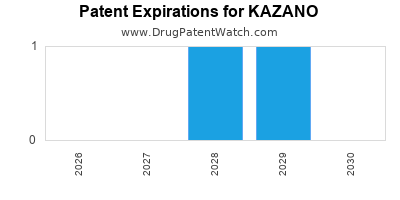

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for KAZANO
Generic Entry Date for KAZANO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for KAZANO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Takeda | Phase 3 |
Pharmacology for KAZANO
| Drug Class | Biguanide Dipeptidyl Peptidase 4 Inhibitor |
| Mechanism of Action | Dipeptidyl Peptidase 4 Inhibitors |
Paragraph IV (Patent) Challenges for KAZANO
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| KAZANO | Tablets | alogliptin benzoate; metformin hydrochloride | 12.5 mg/500 mg and 12.5 mg/1000 mg | 203414 | 3 | 2017-01-25 |
US Patents and Regulatory Information for KAZANO
KAZANO is protected by four US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of KAZANO is ⤷ Get Started Free.
This potential generic entry date is based on patent 8,900,638.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Expired US Patents for KAZANO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Takeda Pharms Usa | KAZANO | alogliptin benzoate; metformin hydrochloride | TABLET;ORAL | 203414-002 | Jan 25, 2013 | 6,303,640 | ⤷ Get Started Free |
| Takeda Pharms Usa | KAZANO | alogliptin benzoate; metformin hydrochloride | TABLET;ORAL | 203414-001 | Jan 25, 2013 | 6,211,205 | ⤷ Get Started Free |
| Takeda Pharms Usa | KAZANO | alogliptin benzoate; metformin hydrochloride | TABLET;ORAL | 203414-002 | Jan 25, 2013 | 7,078,381 | ⤷ Get Started Free |
| Takeda Pharms Usa | KAZANO | alogliptin benzoate; metformin hydrochloride | TABLET;ORAL | 203414-002 | Jan 25, 2013 | 8,288,539 | ⤷ Get Started Free |
| Takeda Pharms Usa | KAZANO | alogliptin benzoate; metformin hydrochloride | TABLET;ORAL | 203414-001 | Jan 25, 2013 | 6,166,043 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for KAZANO
When does loss-of-exclusivity occur for KAZANO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 7557
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 08276842
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0814299
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 94620
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1801351
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 60301
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 267
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 010000028
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 109979
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 0870
Estimated Expiration: ⤷ Get Started Free
Patent: 1070164
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 85138
Patent: PREPARATION SOLIDE COMPRENANT DU ALOGLIPTIN ET METFORMIN CHLORHYDRATE (SOLID PREPARATION COMPRISING ALOGLIPTIN AND METFORMIN HYDROCHLORIDE)
Estimated Expiration: ⤷ Get Started Free
Georgia, Republic of
Patent: 0125672
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 3171
Patent: תכשיר מוצק הכולל אלוגליפטין ומטפורמין הידרוכלוריד (Solid preparation comprising alogliptin and metformin hydrochloride)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 79318
Estimated Expiration: ⤷ Get Started Free
Patent: 10533643
Estimated Expiration: ⤷ Get Started Free
Patent: 14058547
Patent: SOLID PREPARATION COMPRISING ALOGLIPTIN AND METFORMIN HYDROCHLORIDE
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 72
Patent: مستحضر صلب يشمل ألوجليبتين وميتفورمين هيدروكلوريد (SOLID PREPARATION COMPRISING ALOGLIPTIN AND METFORMIN HYDROCHLORIDE)
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 9203
Patent: SOLID PREPARATION COMPRISING ALOGLIPTIN AND METFORMIN HYDROCHLORIDE
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 592
Patent: PREPARATION SOLIDE COMPRENANT L'ALOGLIPTINE ET LE CHLORHYDRATE DE METFORMINE
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 3346
Patent: SOLID PREPARATION COMPRISING ALOGLIPTIN AND METFORMIN HYDROCHLORIDE
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 090882
Patent: PREPARACION SOLIDA QUE COMPRENDE ALOGLIPTINA Y CLORHIDRATO DE METFORMINA
Estimated Expiration: ⤷ Get Started Free
Patent: 140923
Patent: PREPARACION SOLIDA QUE COMPRENDE ALOGLIPTINA Y CLORHIDRATO DE METFORMINA
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1000831
Patent: SOLID PREPARATION COMPRISING ALOGLIPTIN AND METFORMIN HYDROCHLORIDE
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1536786
Estimated Expiration: ⤷ Get Started Free
Patent: 100036367
Patent: SOLID PREPARATION COMPRISING ALOGLIPTIN AND METFORMIN HYDROCHLORIDE
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 03879
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 21102
Estimated Expiration: ⤷ Get Started Free
Patent: 0914066
Patent: Solid preparation
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 10000019
Patent: SOLID PREPARATION COMPRISING ALOGLIPTIN AND METFORMIN HYDROCHLORIDE
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 799
Patent: ТВЕРДЫЙ ПРЕПАРАТ, ВКЛЮЧАЮЩИЙ АЛОГЛИПТИН И ГИДРОХЛОРИД МЕТФОРМИНА;ТВЕРДИЙ ПРЕПАРАТ, ЩО ВКЛЮЧАЄ АЛОГЛІПТИН І ГІДРОХЛОРИД МЕТФОРМІНУ (SOLID PREPARATION COMPRISING ALOGLIPTIN AND METFORMIN HYDROCHLORIDE)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering KAZANO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Tunisia | 2010000019 | SOLID PREPARATION COMPRISING ALOGLIPTIN AND METFORMIN HYDROCHLORIDE | ⤷ Get Started Free |
| European Patent Office | 1520582 | ⤷ Get Started Free | |
| Denmark | 1174135 | ⤷ Get Started Free | |
| Eurasian Patent Organization | 013427 | ИНГИБИТОРЫ ДИПЕПТИДИЛПЕПТИДАЗЫ (DIPEPTIDYL PEPTIDASE INHIBITORS) | ⤷ Get Started Free |
| Russian Federation | 2223760 | СПОСОБЫ ПРОФИЛАКТИКИ И ЛЕЧЕНИЯ, ПРИМЕНЕНИЕ УСИЛИТЕЛЕЙ ЧУВСТВИТЕЛЬНОСТИ К ИНСУЛИНУ (METHOD FOR PROPHYLAXIS AND TREATMENT, USING ENHANCER FOR INSULIN SENSIBILITY) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for KAZANO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1586571 | 132014902238640 | Italy | ⤷ Get Started Free | PRODUCT NAME: ALOGLIPTIN IN TUTTE LE FORME PROTETTE DAL BREVETTO DI BASE(VIPIDIA); AUTHORISATION NUMBER(S) AND DATE(S): DA EU/1/13/844/001 A EU/1/13/844/027, 20130923 |
| 1084705 | PA2014043 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: SAXAGLIPTINUM; REGISTRATION NO/DATE: EU/1/09/545/001-015 20091001 |
| 1084705 | C300709 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: ALOGLIPTIN; REGISTRATION NO/DATE: EU/1/13/844/001-027 20130919 |
| 1586571 | 92374 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: ALOGLIPTIN SOUS TOUTES SES FORMES TELLES QUE PROTEGEES PAR LE BREVET DE BASE. FIRST REGISTRATION: 20130923 |
| 1084705 | CR 2014 00062 | Denmark | ⤷ Get Started Free | PRODUCT NAME: SAXAGLIPTIN OG FARMACEUTISK SALTE DERAF, HERUNDER SAXAGLIPTIN HYDROCHLORID; REG. NO/DATE: EU/1/09/545/001-015 20091005 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for KAZANO
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
