PROMACTA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Promacta, and what generic alternatives are available?
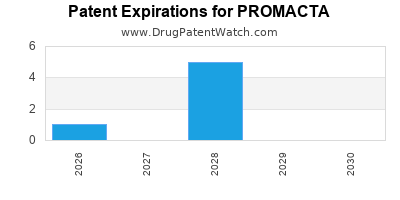
Promacta is a drug marketed by Novartis and is included in two NDAs. There are six patents protecting this drug and two Paragraph IV challenges.
This drug has one hundred and thirty-four patent family members in forty-one countries.
The generic ingredient in PROMACTA is eltrombopag olamine. There are three drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the eltrombopag olamine profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Promacta
A generic version of PROMACTA was approved as eltrombopag olamine by ANNORA PHARMA on April 18th, 2024.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for PROMACTA?
- What are the global sales for PROMACTA?
- What is Average Wholesale Price for PROMACTA?
Summary for PROMACTA
| International Patents: | 134 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 70 |
| Clinical Trials: | 31 |
| Patent Applications: | 671 |
| Drug Prices: | Drug price information for PROMACTA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PROMACTA |
| What excipients (inactive ingredients) are in PROMACTA? | PROMACTA excipients list |
| DailyMed Link: | PROMACTA at DailyMed |

Recent Clinical Trials for PROMACTA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Food and Drug Administration (FDA) | Phase 1 |
| University of California, San Francisco | Phase 1 |
| University of California, Davis | Phase 1 |
Pharmacology for PROMACTA
Paragraph IV (Patent) Challenges for PROMACTA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| PROMACTA | Tablets | eltrombopag olamine | 12.5 mg and 25 mg | 022291 | 1 | 2014-02-04 |
| PROMACTA | Tablets | eltrombopag olamine | 50 mg and 75 mg | 022291 | 1 | 2014-01-07 |
US Patents and Regulatory Information for PROMACTA
PROMACTA is protected by six US patents and one FDA Regulatory Exclusivity.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | PROMACTA KIT | eltrombopag olamine | FOR SUSPENSION;ORAL | 207027-002 | Sep 27, 2018 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-003 | Sep 8, 2009 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Novartis | PROMACTA KIT | eltrombopag olamine | FOR SUSPENSION;ORAL | 207027-001 | Aug 24, 2015 | AB | RX | Yes | Yes | 7,547,719*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-005 | Nov 16, 2012 | DISCN | Yes | No | 7,547,719*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-004 | Oct 20, 2011 | AB | RX | Yes | No | 8,828,430*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PROMACTA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-003 | Sep 8, 2009 | 7,452,874*PED | ⤷ Get Started Free |
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-005 | Nov 16, 2012 | 7,795,293*PED | ⤷ Get Started Free |
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-005 | Nov 16, 2012 | 7,790,704*PED | ⤷ Get Started Free |
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-001 | Nov 20, 2008 | 7,452,874*PED | ⤷ Get Started Free |
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-005 | Nov 16, 2012 | 7,473,686*PED | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for PROMACTA
When does loss-of-exclusivity occur for PROMACTA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 9656
Estimated Expiration: ⤷ Get Started Free
Patent: 7711
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 07352608
Estimated Expiration: ⤷ Get Started Free
Patent: 12201288
Estimated Expiration: ⤷ Get Started Free
Patent: 14202367
Estimated Expiration: ⤷ Get Started Free
Patent: 16202063
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0721651
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 85831
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 07002242
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1686930
Estimated Expiration: ⤷ Get Started Free
Patent: 2688207
Estimated Expiration: ⤷ Get Started Free
Patent: 2697745
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 60058
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 143
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0160206
Estimated Expiration: ⤷ Get Started Free
Patent: 0240595
Estimated Expiration: ⤷ Get Started Free
Patent: 0250383
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 17284
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 52237
Estimated Expiration: ⤷ Get Started Free
Patent: 90730
Estimated Expiration: ⤷ Get Started Free
Patent: 18732
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 009000253
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 077628
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 0883
Estimated Expiration: ⤷ Get Started Free
Patent: 4294
Estimated Expiration: ⤷ Get Started Free
Patent: 0971018
Estimated Expiration: ⤷ Get Started Free
Patent: 1400387
Estimated Expiration: ⤷ Get Started Free
Patent: 1991590
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 52237
Estimated Expiration: ⤷ Get Started Free
Patent: 90730
Estimated Expiration: ⤷ Get Started Free
Patent: 18732
Estimated Expiration: ⤷ Get Started Free
Patent: 18733
Estimated Expiration: ⤷ Get Started Free
Patent: 00104
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 90730
Estimated Expiration: ⤷ Get Started Free
Patent: 18732
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 36968
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 27209
Estimated Expiration: ⤷ Get Started Free
Patent: 67736
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1891
Estimated Expiration: ⤷ Get Started Free
Patent: 8840
Estimated Expiration: ⤷ Get Started Free
Patent: 4602
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 19866
Estimated Expiration: ⤷ Get Started Free
Patent: 35078
Estimated Expiration: ⤷ Get Started Free
Patent: 44713
Estimated Expiration: ⤷ Get Started Free
Patent: 60289
Estimated Expiration: ⤷ Get Started Free
Patent: 42148
Estimated Expiration: ⤷ Get Started Free
Patent: 42149
Estimated Expiration: ⤷ Get Started Free
Patent: 10526140
Estimated Expiration: ⤷ Get Started Free
Patent: 14005302
Estimated Expiration: ⤷ Get Started Free
Patent: 15129195
Estimated Expiration: ⤷ Get Started Free
Patent: 17137343
Estimated Expiration: ⤷ Get Started Free
Patent: 19123747
Estimated Expiration: ⤷ Get Started Free
Patent: 21100968
Estimated Expiration: ⤷ Get Started Free
Patent: 23011888
Estimated Expiration: ⤷ Get Started Free
Patent: 25020367
Estimated Expiration: ⤷ Get Started Free
Patent: 25020368
Estimated Expiration: ⤷ Get Started Free
Patent: 25081605
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 43
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 90730
Estimated Expiration: ⤷ Get Started Free
Patent: 18732
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 8072
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 09011881
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 236
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 0888
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 080773
Estimated Expiration: ⤷ Get Started Free
Patent: 121407
Estimated Expiration: ⤷ Get Started Free
Patent: 151953
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 52237
Estimated Expiration: ⤷ Get Started Free
Patent: 90730
Estimated Expiration: ⤷ Get Started Free
Patent: 18732
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 52237
Estimated Expiration: ⤷ Get Started Free
Patent: 90730
Estimated Expiration: ⤷ Get Started Free
Patent: 18732
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 52237
Estimated Expiration: ⤷ Get Started Free
Patent: 90730
Estimated Expiration: ⤷ Get Started Free
Patent: 18732
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0907710
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1475971
Estimated Expiration: ⤷ Get Started Free
Patent: 1537200
Estimated Expiration: ⤷ Get Started Free
Patent: 1632851
Estimated Expiration: ⤷ Get Started Free
Patent: 100020456
Estimated Expiration: ⤷ Get Started Free
Patent: 140049086
Estimated Expiration: ⤷ Get Started Free
Patent: 150008513
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 65179
Estimated Expiration: ⤷ Get Started Free
Patent: 81985
Estimated Expiration: ⤷ Get Started Free
Patent: 32244
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 39267
Estimated Expiration: ⤷ Get Started Free
Patent: 38674
Estimated Expiration: ⤷ Get Started Free
Patent: 0843742
Estimated Expiration: ⤷ Get Started Free
Patent: 1410240
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 261
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PROMACTA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Hong Kong | 1056114 | ⤷ Get Started Free | |
| Japan | 6144713 | ⤷ Get Started Free | |
| Croatia | P20240595 | ⤷ Get Started Free | |
| Denmark | 2152237 | ⤷ Get Started Free | |
| Norway | 20025566 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PROMACTA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1294378 | C300451 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: ELTROMBOPAG, DESGEWENST IN DE; REGISTRATION NO/DATE: EU/1/10/612/001-006 20100311 |
| 1294378 | C201000022 | Spain | ⤷ Get Started Free | PRODUCT NAME: ELTROMBOPAG; NATIONAL AUTHORISATION NUMBER: EU/1/10/612/001-006; DATE OF AUTHORISATION: 20100315; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/10/612/001-006; DATE OF FIRST AUTHORISATION IN EEA: 20100315 |
| 1294378 | C01294378/01 | Switzerland | ⤷ Get Started Free | FORMER OWNER: GLAXOSMITHKLINE LLC, US |
| 1294378 | SPC020/2010 | Ireland | ⤷ Get Started Free | SPC020/2010: 20110308, EXPIRES: 20250310 |
| 1534390 | 91 3-2010 | Slovakia | ⤷ Get Started Free | PRODUCT NAME: ELTROMBOPAG; REGISTRATION NO/DATE: EU/1/10/612/001 - EU/1/10/612/006 20100315 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for PROMACTA (Eltrombopag)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
