EDARBI Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Edarbi, and what generic alternatives are available?
Edarbi is a drug marketed by Azurity and is included in one NDA. There are three patents protecting this drug and one Paragraph IV challenge.
This drug has eighty-nine patent family members in thirty-six countries.
The generic ingredient in EDARBI is azilsartan kamedoxomil. There are six drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the azilsartan kamedoxomil profile page.
DrugPatentWatch® Generic Entry Outlook for Edarbi
Edarbi was eligible for patent challenges on February 25, 2015.
There have been six patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for EDARBI?
- What are the global sales for EDARBI?
- What is Average Wholesale Price for EDARBI?
Summary for EDARBI
| International Patents: | 89 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 65 |
| Clinical Trials: | 16 |
| Patent Applications: | 427 |
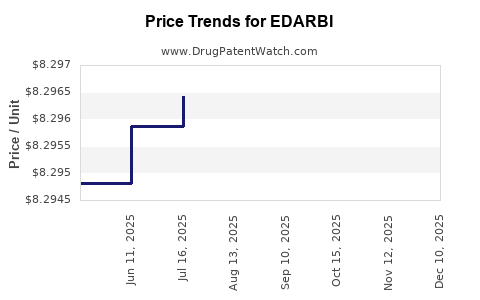
| Drug Prices: | Drug price information for EDARBI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for EDARBI |
| What excipients (inactive ingredients) are in EDARBI? | EDARBI excipients list |
| DailyMed Link: | EDARBI at DailyMed |
Recent Clinical Trials for EDARBI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Gedeon Richter Plc. | PHASE1 |
| Takeda | |
| Takeda | Phase 1 |
Pharmacology for EDARBI
| Drug Class | Angiotensin 2 Receptor Blocker |
| Mechanism of Action | Angiotensin 2 Type 1 Receptor Antagonists |
| Physiological Effect | Decreased Blood Pressure |
Paragraph IV (Patent) Challenges for EDARBI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| EDARBI | Tablets | azilsartan kamedoxomil | 40 mg and 80 mg | 200796 | 1 | 2020-04-10 |
US Patents and Regulatory Information for EDARBI
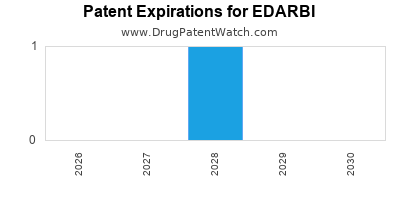
EDARBI is protected by three US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azurity | EDARBI | azilsartan kamedoxomil | TABLET;ORAL | 200796-001 | Feb 25, 2011 | RX | Yes | No | 7,572,920 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Azurity | EDARBI | azilsartan kamedoxomil | TABLET;ORAL | 200796-002 | Feb 25, 2011 | RX | Yes | Yes | 7,572,920 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Azurity | EDARBI | azilsartan kamedoxomil | TABLET;ORAL | 200796-001 | Feb 25, 2011 | RX | Yes | No | 9,066,936 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Azurity | EDARBI | azilsartan kamedoxomil | TABLET;ORAL | 200796-001 | Feb 25, 2011 | RX | Yes | No | 7,157,584 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for EDARBI
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Azurity | EDARBI | azilsartan kamedoxomil | TABLET;ORAL | 200796-002 | Feb 25, 2011 | 7,572,920 | ⤷ Get Started Free |
| Azurity | EDARBI | azilsartan kamedoxomil | TABLET;ORAL | 200796-002 | Feb 25, 2011 | 5,958,961 | ⤷ Get Started Free |
| Azurity | EDARBI | azilsartan kamedoxomil | TABLET;ORAL | 200796-001 | Feb 25, 2011 | 5,583,141 | ⤷ Get Started Free |
| Azurity | EDARBI | azilsartan kamedoxomil | TABLET;ORAL | 200796-001 | Feb 25, 2011 | 7,572,920 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for EDARBI
When does loss-of-exclusivity occur for EDARBI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 5850
Patent: COMPOSICION FARMACEUTICA SOLIDA QUE COMPRENDE UN DERIVADO DE BENZIMIDAZOL-7-CARBOXILATO Y UN AGENTE DE CONTROL DE PH
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 08235790
Patent: Solid pharmaceutical composition comprising a benzimidazole-7-carboxylate derivative and a pH control agent
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0809522
Patent: COMPOSIÇÃO FARMACÊUTICA SÓLIDA, MÉTODOS PARA ESTABILIZAR UM COMPOSTO, E PARA MELHORAR DISSOLUÇÃO DE UM COMPOSTO, E, USO DE UM AGENTE DE CONTROLE DE PH.
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 81143
Patent: COMPOSITION PHARMACEUTIQUE SOLIDE COMPRENANT UN DERIVE DE BENZIMIDAZOLE-7-CARBOXYLATE ET UN AGENT DE CONTROLE DU PH (SOLID PHARMACEUTICAL COMPOSITION COMPRISING A BENZIMIDAZOLE-7-CARBOXYLATE DERIVATIVE AND A PH CONTROL AGENT)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 08000868
Patent: COMPOSICION FARMACEUTICA SOLIDA QUE COMPRENDE UN COMPUESTO DERIVADO DE BENZIMIDAZOL Y UN AGENTE DE CONTROL DE PH; METODO DE ESTABILIZACION Y DE MEJORAMIENTO DE LA DISOLUCION; USO DE UN AGENTE DE CONTROL DE PH.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1677961
Patent: Solid pharmaceutical composition comprising a benzimidazole-7-carboxylate derivative and a ph control agent
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 6593
Patent: ТВЕРДАЯ ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ, ВКЛЮЧАЮЩАЯ ПРОИЗВОДНОЕ БЕНЗИМИДАЗОЛ-7-КАРБОКСИЛАТА И pH РЕГУЛИРУЮЩИЙ АГЕНТ (SOLID PHARMACEUTICAL COMPOSITION COMPRISING A BENZIMIDAZOLE-7-CARBOXYLATE DERIVATIVE AND A PH CONTROL AGENT)
Estimated Expiration: ⤷ Get Started Free
Patent: 0970896
Patent: ТВЕРДАЯ ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ, ВКЛЮЧАЮЩАЯ ПРОИЗВОДНОЕ БЕНЗИМИДАЗОЛ-7-КАРБОКСИЛАТА И pH РЕГУЛИРУЮЩИЙ АГЕНТ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 24903
Patent: COMPOSITION PHARMACEUTIQUE SOLIDE COMPRENANT UN DÉRIVÉ DE BENZIMIDAZOLE-7-CARBOXYLATE ET UN AGENT DE CONTRÔLE DU PH (SOLID PHARMACEUTICAL COMPOSITION COMPRISING A BENZIMIDAZOLE-7-CARBOXYLATE DERIVATIVE AND A PH CONTROL AGENT)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 83632
Estimated Expiration: ⤷ Get Started Free
Patent: 10522692
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 09010167
Patent: COMPOSICION FARMACEUTICA SOLIDA QUE COMPRENDE UN DERIVADO DE BENCIMIDAZOL-7-CARBOXILATO Y UN AGENTE PARA EL CONTROL DEL PH. (SOLID PHARMACEUTICAL COMPOSITION COMPRISING A BENZIMIDAZOLE-7-CARBOXYLATE DERIVATIVE AND A PH CONTROL AGENT.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 9851
Patent: SOLID PHARMACEUTICAL COMPOSITION COMPRISING A BENZIMIDAZOLE-7-CARBOXYLATE DERIVATIVE AND A PH CONTROL AGENT
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 090550
Patent: COMPOSICION FARMACEUTICA SOLIDA QUE COMPRENDE UN DERIVADO DE BENCIMIDAZOL Y UN AGENTE DE CONTROL DE PH
Estimated Expiration: ⤷ Get Started Free
Patent: 130210
Patent: COMPOSICION FARMACEUTICA SOLIDA QUE COMPRENDE UN DERIVADO DE BENCIMIDAZOL Y UN AGENTE DE CONTROL DE PH
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 24903
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 090125846
Patent: SOLID PHARMACEUTICAL COMPOSITION COMPRISING A BENZIMIDAZOLE-7-CARBOXYLATE DERIVATIVE AND A PH CONTROL AGENT
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 43784
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 15634
Estimated Expiration: ⤷ Get Started Free
Patent: 0902089
Patent: Solid pharmaceutical composition
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering EDARBI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Russian Federation | 2168510 | ГЕТЕРОЦИКЛИЧЕСКОЕ СОЕДИНЕНИЕ, ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ, СПОСОБ АНТАГОНИЗИРОВАНИЯ АНГИОТЕНЗИНА II (HETEROCYCLIC COMPOUND, PHARMACEUTICAL COMPOSITION, METHOD OF ANTAGONISM OF ANGIOTENSIN-II) | ⤷ Get Started Free |
| Mexico | 9203627 | COMPUESTOS HETEROCICLICOS Y PROCESO PARA SU PRODUCCION. | ⤷ Get Started Free |
| Croatia | P20090593 | ⤷ Get Started Free | |
| China | 100503605 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for EDARBI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1718641 | C300525 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: AZILSARTAN MEDOXOMIL, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCHE AANVAARDBAAR ZOUT, IN HET BIJZONDER HET KALIUMZOUT; REGISTRATION NO/DATE: EU/1/11/734/001-011EU/1/11/735/001-011 2011071207 |
| 2119715 | 2018/006 | Ireland | ⤷ Get Started Free | PRODUCT NAME: COMBINATION OF AZILSARTAN MEDOXOMIL AND CHLORTALIDONE (EDARBYCLOR); NAT REGISTRATION NO/DATE: PA/2167/001/001-002 20170804; FIRST REGISTRATION NO/DATE: 63145 01-02 20141028 |
| 1718641 | 12C0034 | France | ⤷ Get Started Free | PRODUCT NAME: AZILSARTAN MEDOXOMIL ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/11/735/001 20111207 |
| 1718641 | 91962 | Luxembourg | ⤷ Get Started Free | 91962, EXPIRES: 20261207 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for EDARBI (Erdosteine): An Analytical Overview
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
