OPSUMIT Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Opsumit, and when can generic versions of Opsumit launch?
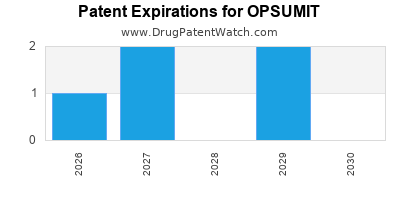
Opsumit is a drug marketed by Actelion and is included in one NDA. There are five patents protecting this drug and one Paragraph IV challenge.
This drug has ninety-nine patent family members in thirty-four countries.
The generic ingredient in OPSUMIT is macitentan. There are ten drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the macitentan profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Opsumit
A generic version of OPSUMIT was approved as macitentan by ALEMBIC on August 18th, 2025.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for OPSUMIT?
- What are the global sales for OPSUMIT?
- What is Average Wholesale Price for OPSUMIT?
Summary for OPSUMIT
| International Patents: | 99 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 17 |
| Patent Applications: | 984 |
| Drug Prices: | Drug price information for OPSUMIT |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for OPSUMIT |
| What excipients (inactive ingredients) are in OPSUMIT? | OPSUMIT excipients list |
| DailyMed Link: | OPSUMIT at DailyMed |

Recent Clinical Trials for OPSUMIT
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| American Heart Association | Phase 4 |
| Janssen, LP | Phase 4 |
| Janssen Pharmaceutical K.K. | Phase 3 |
Pharmacology for OPSUMIT
| Drug Class | Endothelin Receptor Antagonist |
| Mechanism of Action | Endothelin Receptor Antagonists |
Paragraph IV (Patent) Challenges for OPSUMIT
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| OPSUMIT | Tablets | macitentan | 10 mg | 204410 | 11 | 2017-10-18 |
US Patents and Regulatory Information for OPSUMIT
OPSUMIT is protected by five US patents and two FDA Regulatory Exclusivities.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actelion | OPSUMIT | macitentan | TABLET;ORAL | 204410-001 | Oct 18, 2013 | AB | RX | Yes | Yes | 8,268,847*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Actelion | OPSUMIT | macitentan | TABLET;ORAL | 204410-001 | Oct 18, 2013 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Actelion | OPSUMIT | macitentan | TABLET;ORAL | 204410-001 | Oct 18, 2013 | AB | RX | Yes | Yes | 9,265,762*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Actelion | OPSUMIT | macitentan | TABLET;ORAL | 204410-001 | Oct 18, 2013 | AB | RX | Yes | Yes | 7,094,781*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Actelion | OPSUMIT | macitentan | TABLET;ORAL | 204410-001 | Oct 18, 2013 | AB | RX | Yes | Yes | 8,367,685*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for OPSUMIT
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Janssen-Cilag International N.V. | Opsumit | macitentan | EMEA/H/C/002697Opsumit, as monotherapy or in combination, is indicated for the long-term treatment of pulmonary arterial hypertension (PAH) in adult patients of WHO Functional Class (FC) II to III.Efficacy has been shown in a PAH population including idiopathic and heritable PAH, PAH associated with connective tissue disorders, and PAH associated with corrected simple congenital heart disease. | Authorised | no | no | yes | 2013-12-20 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for OPSUMIT
When does loss-of-exclusivity occur for OPSUMIT?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2501
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 07290099
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0715698
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 59770
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 07002494
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1511365
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0131233
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 14735
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 59246
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 59246
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 0240045
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1054
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 33597
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 400046
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 7235
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 08113
Estimated Expiration: ⤷ Get Started Free
Patent: 10502588
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 4591
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 09002057
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 704
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 1308
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 5702
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 2554
Estimated Expiration: ⤷ Get Started Free
Patent: 24059
Estimated Expiration: ⤷ Get Started Free
Patent: 091254
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 59246
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 59246
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 62249
Estimated Expiration: ⤷ Get Started Free
Patent: 09111378
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 59246
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0902164
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1473022
Estimated Expiration: ⤷ Get Started Free
Patent: 090057009
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 38792
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 88556
Estimated Expiration: ⤷ Get Started Free
Patent: 0823198
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering OPSUMIT around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2010502588 | ⤷ Get Started Free | |
| Austria | 323079 | ⤷ Get Started Free | |
| Russian Federation | 2008113869 | ⤷ Get Started Free | |
| Portugal | 2059246 | ⤷ Get Started Free | |
| China | 100432070 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for OPSUMIT
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1345920 | CA 2014 00012 | Denmark | ⤷ Get Started Free | PRODUCT NAME: MACITENTAN OG FARMACEUTISK ACCEPTABLE SALTE HERAF; REG. NO/DATE: EU/1/13/893 20131220 |
| 2059246 | LUC00371 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: A COMBINATION OF (A) MACITENTAN OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF AND (B) TADALAFIL OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; AUTHORISATION NUMBER AND DATE: EU/1/24/1859 20240930 |
| 2059246 | 832 | Finland | ⤷ Get Started Free | |
| 1345920 | 2014/018 | Ireland | ⤷ Get Started Free | PRODUCT NAME: MACITENTAN, THE STEREOISOMERS AND THE PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF.; REGISTRATION NO/DATE: EU/1/13/893 20131220 |
| 1345920 | 14C0017 | France | ⤷ Get Started Free | PRODUCT NAME: MACITENTAN ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/13/893 20131220 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for Opsumit (Macitentan)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
