ALIQOPA Drug Patent Profile
✉ Email this page to a colleague
When do Aliqopa patents expire, and when can generic versions of Aliqopa launch?
Aliqopa is a drug marketed by Bayer Healthcare and is included in one NDA. There are three patents protecting this drug.
This drug has one hundred and five patent family members in forty-eight countries.
The generic ingredient in ALIQOPA is copanlisib dihydrochloride. Additional details are available on the copanlisib dihydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Aliqopa
Aliqopa was eligible for patent challenges on September 14, 2021.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 29, 2032. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ALIQOPA?
- What are the global sales for ALIQOPA?
- What is Average Wholesale Price for ALIQOPA?
Summary for ALIQOPA
| International Patents: | 105 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 73 |
| Clinical Trials: | 16 |
| Drug Prices: | Drug price information for ALIQOPA |
| What excipients (inactive ingredients) are in ALIQOPA? | ALIQOPA excipients list |
| DailyMed Link: | ALIQOPA at DailyMed |
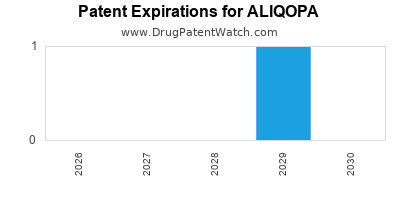

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ALIQOPA
Generic Entry Date for ALIQOPA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
POWDER;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ALIQOPA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| NRG Oncology | Phase 2 |
| City of Hope Medical Center | Phase 1/Phase 2 |
| AbbVie | Phase 1/Phase 2 |
US Patents and Regulatory Information for ALIQOPA
ALIQOPA is protected by three US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ALIQOPA is ⤷ Get Started Free.
This potential generic entry date is based on patent 10,383,876.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayer Healthcare | ALIQOPA | copanlisib dihydrochloride | POWDER;INTRAVENOUS | 209936-001 | Sep 14, 2017 | DISCN | Yes | No | 9,636,344 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Bayer Healthcare | ALIQOPA | copanlisib dihydrochloride | POWDER;INTRAVENOUS | 209936-001 | Sep 14, 2017 | DISCN | Yes | No | RE46856 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Bayer Healthcare | ALIQOPA | copanlisib dihydrochloride | POWDER;INTRAVENOUS | 209936-001 | Sep 14, 2017 | DISCN | Yes | No | 10,383,876 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ALIQOPA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bayer Healthcare | ALIQOPA | copanlisib dihydrochloride | POWDER;INTRAVENOUS | 209936-001 | Sep 14, 2017 | 7,511,041 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ALIQOPA
When does loss-of-exclusivity occur for ALIQOPA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 09
Patent: SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS
Estimated Expiration: ⤷ Get Started Free
Argentina
Patent: 5718
Patent: SALES DE 2,3-DIHIDROIMIDAZO[1,2-C]QUINAZOLINA SUBSTITUIDA
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 12238891
Patent: Substituted 2,3-dihydroimidazo[1,2-c]quinazoline salts
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2013025549
Patent: sais de 2,3-hidroimidazo[1,2-c]quinazolina substituídos
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 32123
Patent: SELS DE 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SUBSTITUES (SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 13002870
Patent: Sal diclorhidrato de 2-amino-n-[7-metoxi-8-(3-morfolin-4-ilpropoxi)-2,3-dihidroimidazo[1,2-c]quinazolin-5-il]pirimidin-5-carboxamida; método de preparación; composición farmacéutica; combinación farmacéutica; y su uso para el tratamiento o profilaxis del cáncer.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 3649091
Patent: Substituted 2,3-dihydroimidazo[1,2-c]quinazoline salts
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 81534
Patent: Sales de 2,3-dihidroimidazol[1,2-c]quinazolina substituida
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 130511
Patent: SALES DE 2,3-DIHIDROIMIDAZO[1,2-C]QUINAZOLINA SUBSTITUIDA
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0150138
Estimated Expiration: ⤷ Get Started Free
Cuba
Patent: 208
Patent: SAL DE DICLORHIDRATO DE 2-AMINO-N-[7-METOXI-8-(3-MORFOLIN-4-ILPROPOXI)-2,3-DIHIDROIMIDAZO-[1,2-C]QUINAZOLIN-5-IL]PIRIMIDIN-5-CARBOXAMIDA Y MÉTODO DE PREPARACIÓN DE LA MISMA
Estimated Expiration: ⤷ Get Started Free
Patent: 130133
Patent: SALES DE 2,3- DIHIDROIMIDAZO[1,2- C] QUINAZOLINAS SUSTITUDA
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 16231
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 94508
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 013000223
Patent: SALES DE 2,3-DIHIDROIMIDAZO[1,2-C]QUINAZOLINA SUBSTITUIDA.
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 13013006
Patent: SALES DE 2,3-DIHIDROIMIDAZO[1,2-C]QUINAZOLINA SUBSTITUIDA
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 3646
Patent: ЗАМЕЩЁННЫЕ СОЛИ 2,3-ДИГИДРОИМИДАЗО[1,2-C]ХИНАЗОЛИНА (SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-c]QUINAZOLINE SALTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 1391470
Patent: ЗАМЕЩЕННЫЕ СОЛИ 2,3-ДИГИДРОИМИДАЗО[1,2-C]ХИНАЗОЛИНА
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 08525
Patent: Sels de 2,3-dihydroimidazo[1,2-C]quinazoline substitués (Substituted 2,3-dihydroimidazo[1,2-c]quinazoline salts)
Estimated Expiration: ⤷ Get Started Free
Patent: 94508
Patent: SELS DE 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SUBSTITUÉS (SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS)
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 1300234
Patent: SALES DE 2,3-DIHIDROIMIDAZO[1,2-C]QUINAZOLINA SUSTITUIDA
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 95907
Patent: 取代的 -二氫咪唑並 喹唑啉鹽 (SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS 23-[12-C])
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 8561
Patent: מלחים 3,2-דיהידרואימידאזו[2,1-c]קווינאזולין מותמרים (Substituted 2,3 -dihydroimidazo[1,2-c]quinazoline salts)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 63834
Estimated Expiration: ⤷ Get Started Free
Patent: 26961
Estimated Expiration: ⤷ Get Started Free
Patent: 14510119
Estimated Expiration: ⤷ Get Started Free
Patent: 15164936
Patent: 置換2,3−ジヒドロイミダゾ[1,2−C]キナゾリン塩類 (SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS)
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 58
Patent: أملاح 3،2- دايهيدروإيميدازو[2،1C-] كوينازولين مستبدل (SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS)
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 9452
Patent: SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 6057
Patent: SALES DE 2,3-DIHIDROIMIDAZO[1,2-C]QUINAZOLINA SUBSTITUIDA. (SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 13011583
Patent: SALES DE 2,3-DIHIDROIMIDAZO[1,2-C]QUINAZOLINA SUBSTITUIDA. (SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS.)
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 021
Patent: SUPSTITUISANE SOLI 2,3-DIHIDROIMIDAZO[1,2-C]HINAZOLINA (SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 014
Patent: SELS DE 2,3-DIHYDROIMIDAZO [1,2-C]QUINAZOLINE SUBSTITUÉS
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 6198
Patent: Substituted 2,3-dihydroimidazo[1,2-c]quinazoline salts
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 141038
Patent: SALES DE 2,3-DIHIDROIMIDAZO[1,2-C]QUINAZOLINA SUBSTITUIDA
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 013502065
Patent: SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 94508
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 94508
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01500037
Patent: Sali di 2,3-diidroimidazoÄ1,2-cÜchinazolina sostituiti
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 811
Patent: SUPSTITUISANE SOLI 2,3-DIHIDROIMIDAZO[1,2-C]HINAZOLINA (SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 3595
Patent: SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 94508
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1307105
Patent: SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1937501
Estimated Expiration: ⤷ Get Started Free
Patent: 140021637
Patent: SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 29653
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 49954
Estimated Expiration: ⤷ Get Started Free
Patent: 92158
Estimated Expiration: ⤷ Get Started Free
Patent: 1249847
Patent: Substituted 2,3-dihydroimidazo[1,2-c]quinazoline salts
Estimated Expiration: ⤷ Get Started Free
Patent: 1637656
Patent: Substituted 2,3-dihydroimidazo[1,2-C]quinazoline salts
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 13000401
Patent: SUBSTITUTED 2,3-DIHYDROIMIDAZO[1,2-C]QUINAZOLINE SALTS
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 1604
Patent: ЗАМІЩЕНІ 2,3-ДИГІДРОІМІДАЗО[1,2-c]ХІНАЗОЛІНОВІ СОЛІ
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 985
Patent: SALES DE 2,3-DIHIDROIMIDAZO [1,2-C] QUINAZOLINA SUSTITUIDA
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ALIQOPA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Panama | 8759601 | ⤷ Get Started Free | |
| European Patent Office | 2508525 | Sels de 2,3-dihydroimidazo[1,2-C]quinazoline substitués (Substituted 2,3-dihydroimidazo[1,2-c]quinazoline salts) | ⤷ Get Started Free |
| Taiwan | I406662 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for Aliqopa (Copanlisib)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
