WINLEVI Drug Patent Profile
✉ Email this page to a colleague
When do Winlevi patents expire, and when can generic versions of Winlevi launch?
Winlevi is a drug marketed by Sun Pharm and is included in one NDA. There are ten patents protecting this drug and one Paragraph IV challenge.
This drug has ninety patent family members in twenty-seven countries.
The generic ingredient in WINLEVI is clascoterone. One supplier is listed for this compound. Additional details are available on the clascoterone profile page.
DrugPatentWatch® Generic Entry Outlook for Winlevi
Winlevi was eligible for patent challenges on August 26, 2024.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be July 25, 2030. This may change due to patent challenges or generic licensing.
There have been two patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for WINLEVI?
- What are the global sales for WINLEVI?
- What is Average Wholesale Price for WINLEVI?
Summary for WINLEVI
| International Patents: | 90 |
| US Patents: | 10 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 51 |
| Clinical Trials: | 5 |
| Patent Applications: | 172 |
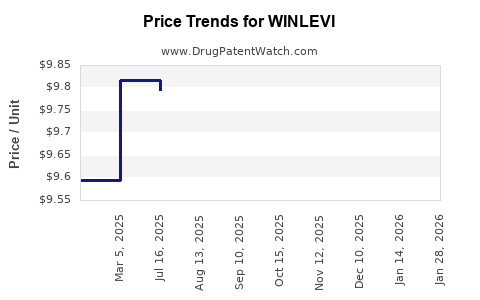
| Drug Prices: | Drug price information for WINLEVI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for WINLEVI |
| What excipients (inactive ingredients) are in WINLEVI? | WINLEVI excipients list |
| DailyMed Link: | WINLEVI at DailyMed |
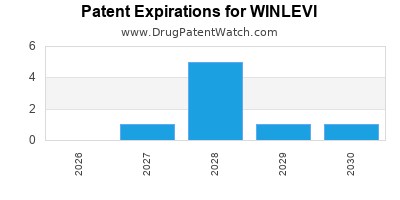
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for WINLEVI
Generic Entry Date for WINLEVI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CREAM;TOPICAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for WINLEVI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Narrows Institute for Biomedical Research | PHASE2 |
| Sun Pharmaceutical Industries Limited | PHASE2 |
| Sun Pharmaceutical Industries Limited | PHASE4 |
Pharmacology for WINLEVI
| Drug Class | Androgen Receptor Inhibitor |
| Mechanism of Action | Androgen Receptor Antagonists |
Paragraph IV (Patent) Challenges for WINLEVI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| WINLEVI | Cream | clascoterone | 1% | 213433 | 1 | 2024-08-26 |
US Patents and Regulatory Information for WINLEVI
WINLEVI is protected by ten US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of WINLEVI is ⤷ Get Started Free.
This potential generic entry date is based on patent 8,785,427.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sun Pharm | WINLEVI | clascoterone | CREAM;TOPICAL | 213433-001 | Aug 26, 2020 | RX | Yes | Yes | 12,337,002 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Sun Pharm | WINLEVI | clascoterone | CREAM;TOPICAL | 213433-001 | Aug 26, 2020 | RX | Yes | Yes | 9,433,628 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Sun Pharm | WINLEVI | clascoterone | CREAM;TOPICAL | 213433-001 | Aug 26, 2020 | RX | Yes | Yes | 8,143,240 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for WINLEVI
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sun Pharm | WINLEVI | clascoterone | CREAM;TOPICAL | 213433-001 | Aug 26, 2020 | 8,865,690 | ⤷ Get Started Free |
| Sun Pharm | WINLEVI | clascoterone | CREAM;TOPICAL | 213433-001 | Aug 26, 2020 | 9,211,295 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for WINLEVI
When does loss-of-exclusivity occur for WINLEVI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2235
Estimated Expiration: ⤷ Get Started Free
Patent: 1202
Estimated Expiration: ⤷ Get Started Free
Patent: 1351
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 08285784
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0814163
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 91445
Estimated Expiration: ⤷ Get Started Free
Patent: 71025
Estimated Expiration: ⤷ Get Started Free
Patent: 71039
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1743316
Estimated Expiration: ⤷ Get Started Free
Patent: 3450304
Estimated Expiration: ⤷ Get Started Free
Patent: 4861023
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0140421
Estimated Expiration: ⤷ Get Started Free
Patent: 0151174
Estimated Expiration: ⤷ Get Started Free
Patent: 0151298
Estimated Expiration: ⤷ Get Started Free
Patent: 0191143
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 73891
Estimated Expiration: ⤷ Get Started Free
Patent: 03004
Estimated Expiration: ⤷ Get Started Free
Patent: 03005
Estimated Expiration: ⤷ Get Started Free
Patent: 66175
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 73891
Estimated Expiration: ⤷ Get Started Free
Patent: 03004
Estimated Expiration: ⤷ Get Started Free
Patent: 03005
Estimated Expiration: ⤷ Get Started Free
Patent: 66175
Estimated Expiration: ⤷ Get Started Free
Patent: 21298
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 26206
Estimated Expiration: ⤷ Get Started Free
Patent: 26507
Estimated Expiration: ⤷ Get Started Free
Patent: 44237
Estimated Expiration: ⤷ Get Started Free
Italy
Patent: 20071616
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 46992
Estimated Expiration: ⤷ Get Started Free
Patent: 08944
Estimated Expiration: ⤷ Get Started Free
Patent: 74645
Estimated Expiration: ⤷ Get Started Free
Patent: 10535173
Estimated Expiration: ⤷ Get Started Free
Patent: 13163683
Estimated Expiration: ⤷ Get Started Free
Patent: 16014045
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 66175
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 3238
Estimated Expiration: ⤷ Get Started Free
Patent: 3701
Estimated Expiration: ⤷ Get Started Free
Patent: 10001256
Estimated Expiration: ⤷ Get Started Free
Patent: 19003639
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 9437
Estimated Expiration: ⤷ Get Started Free
Patent: 0767
Estimated Expiration: ⤷ Get Started Free
Patent: 5953
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 73891
Estimated Expiration: ⤷ Get Started Free
Patent: 03004
Estimated Expiration: ⤷ Get Started Free
Patent: 03005
Estimated Expiration: ⤷ Get Started Free
Patent: 66175
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 73891
Estimated Expiration: ⤷ Get Started Free
Patent: 03004
Estimated Expiration: ⤷ Get Started Free
Patent: 03005
Estimated Expiration: ⤷ Get Started Free
Patent: 66175
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 82190
Patent: КРИСТАЛЛИЧЕСКИЕ ФОРМЫ КОРТЕКСОЛОН-17α-ПРОПИОНАТА И СПОСОБЫ ИХ ПОЛУЧЕНИЯ (CRYSTALLINE FORMS OF CORTEXOLONE-17?-PROPIONATE AND METHOD FOR PREPARING THEM)
Estimated Expiration: ⤷ Get Started Free
Patent: 99452
Patent: КРИСТАЛЛИЧЕСКАЯ СОЛЬВАТНАЯ ФОРМА КОРТЕКСОЛОН-17альфа-ПРОПИОНАТА, СПОСОБ ЕЕ ПОЛУЧЕНИЯ И ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ НА ЕЕ ОСНОВЕ (CRYSTALLINE SOLVATE FORM OF CORTEXOLONE-17alpha-PROPIONATE, A PREPARATION METHOD THEREOF AND A PHARMACEUTICAL COMPOSITION BASED THEREON)
Estimated Expiration: ⤷ Get Started Free
Patent: 10107599
Patent: ФЕРМЕНТАТИВНЫЙ СПОСОБ ПОЛУЧЕНИЯ 17-МОНОЭФИРОВ КОРТЕКСОЛОНА И/ИЛИ ИХ 9,11-ДЕГИДРОПРОИЗВОДНЫХ
Estimated Expiration: ⤷ Get Started Free
Patent: 12113839
Patent: КРИСТАЛЛИЧЕСКАЯ СОЛЬВАТНАЯ ФОРМА КОРТЕКСОЛОН-17АЛЬФА-ПРОПИОНАТА И СПОСОБ ЕЕ ПОЛУЧЕНИЯ
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 310
Patent: ENZIMATSKI POSTUPAK ZA DOBIJANJE KORTEKSOLON-17-ALFA-PROPIONATA U KRISTALNOM OBLIKU III (ENZYMATIC PROCESS FOR OBTAINING CORTEXOLONE-17-ALPHA-PROPIONATE IN CRYSTALLINE FORM III)
Estimated Expiration: ⤷ Get Started Free
Patent: 354
Patent: KORTEKSOLON-17ALFA-PROPIONAT U KRISTALNOM OBLIKU I (CORTEXOLONE-17ALPHA-PROPIONATE IN CRYSTALLINE FORM I)
Estimated Expiration: ⤷ Get Started Free
Patent: 361
Patent: KORTEKSOLON-17ALFA-PROPIONAT U KRISTALNOM HIDRATNOM OBLIKU IV (CORTEXOLONE-17ALPHA-PROPIONATE IN HYDRATE CRYSTALLINE FORM IV)
Estimated Expiration: ⤷ Get Started Free
Patent: 950
Patent: FARMACEUTSKE KOMPOZICIJE KOJE SADRŽE KORTEKSOLON-17-ALFA-PROPIONAT (PHARMACEUTICAL COMPOSITIONS CONTAINING CORTEXOLONE-17-ALPHA-PROPIONATE)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 73891
Estimated Expiration: ⤷ Get Started Free
Patent: 03004
Estimated Expiration: ⤷ Get Started Free
Patent: 03005
Estimated Expiration: ⤷ Get Started Free
Patent: 66175
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1000587
Patent: ENZYMATIC PROCESS FOR OBTAINING 17 ALPHA-MONOESTERS OF CORTEXOLONE AND/OR ITS 9,11-DEHYDRODERIVATIVES
Estimated Expiration: ⤷ Get Started Free
Patent: 1100133
Patent: ENZYMATIC PROCESS FOR OBTAINING 17 ALPHA-MONOESTERS OF CORTEXOLONE AND/OR ITS 9,11-DEHYDRODERIVATIVES
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1495192
Estimated Expiration: ⤷ Get Started Free
Patent: 100044845
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 62946
Estimated Expiration: ⤷ Get Started Free
Patent: 51910
Estimated Expiration: ⤷ Get Started Free
Patent: 54934
Estimated Expiration: ⤷ Get Started Free
Patent: 32326
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1909129
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering WINLEVI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Serbia | 58950 | FARMACEUTSKE KOMPOZICIJE KOJE SADRŽE KORTEKSOLON-17-ALFA-PROPIONAT (PHARMACEUTICAL COMPOSITIONS CONTAINING CORTEXOLONE-17-ALPHA-PROPIONATE) | ⤷ Get Started Free |
| Hungary | E026206 | ⤷ Get Started Free | |
| European Patent Office | 3521298 | FORME CRISTALLINE III DE CORTEXOLONE-17-ALPHA-PROPIONATE ET SON UTILISATION PHARMACEUTIQUE (CORTEXOLONE-17-ALPHA-PROPIONATE CRYSTALLINE FORM III AND ITS PHARMACEUTICAL USE) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for WINLEVI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2503005 | C20250037 | Finland | ⤷ Get Started Free | |
| 2503005 | CA 2025 00047 | Denmark | ⤷ Get Started Free | PRODUCT NAME: CLASCOTERONE AND CRYSTALLINE FORMS THEREOF; REG. NO/DATE: EU/1/25/1927 20251017 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for WINLEVI
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
