Argentina: These 30 Drugs Face Patent Expirations and Generic Entry in 2026 - 2027
DrugPatentWatch® Estimated Loss of Exclusivity Dates in Argentina
Generic Entry Dates in Other Countries
These estimated drug patent expiration dates and generic entry opportunity dates are calculated from analysis of known patents covering drugs. Many factors can influence early or late generic entry. This information is provided as a rough estimate of generic entry potential and should not be used as an independent source. The methodology is described in this blog post.
Summary: Argentina: These 30 Drugs Face Patent Expirations and Generic Entry in 2026 - 2027
| Tradename | Ingredient | Estimated Entry Opportunity Date |
|---|---|---|
| FARYDAK | panobinostat lactate | 2026-06-12 |
| LYNPARZA | olaparib | 2027-10-16 |
| OLYSIO | simeprevir sodium | 2026-07-28 |
| XALKORI | crizotinib | 2026-12-04 |
| BYDUREON | exenatide synthetic | 2026-06-28 |
| EPIDUO | adapalene; benzoyl peroxide | 2027-07-13 |
| EPIDUO FORTE | adapalene; benzoyl peroxide | 2027-07-13 |
| >Tradename | >Ingredient | >Estimated Entry Opportunity Date |
Details: Argentina: These 30 Drugs Face Patent Expirations and Generic Entry in 2026 - 2027
When can FARYDAK (panobinostat lactate) generic drug versions launch?
Generic name: panobinostat lactate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 12, 2026
Generic Entry Controlled by: Argentina Patent 61,297
FARYDAK is a drug marketed by Secura. There are two patents protecting this drug.
This drug has sixty-eight patent family members in forty countries. There has been litigation on patents covering FARYDAK
See drug price trends for FARYDAK.
The generic ingredient in FARYDAK is panobinostat lactate. There is one drug master file entry for this API. Additional details are available on the panobinostat lactate profile page.
When can LYNPARZA (olaparib) generic drug versions launch?
Generic name: olaparib
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: October 16, 2027
Generic Entry Controlled by: Argentina Patent 63,320
LYNPARZA is a drug marketed by Astrazeneca. There are twelve patents protecting this drug. Three tentatively approved generics are ready to enter the market.
This drug has two hundred and fifty-four patent family members in fifty-two countries. There has been litigation on patents covering LYNPARZA
See drug price trends for LYNPARZA.
The generic ingredient in LYNPARZA is olaparib. There are three drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the olaparib profile page.
When can OLYSIO (simeprevir sodium) generic drug versions launch?
Generic name: simeprevir sodium
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: July 28, 2026
Generic Entry Controlled by: Argentina Patent 55,359
OLYSIO is a drug marketed by Janssen Prods. There are nine patents protecting this drug.
This drug has one hundred and forty patent family members in forty-three countries.
See drug price trends for OLYSIO.
The generic ingredient in OLYSIO is simeprevir sodium. There is one drug master file entry for this API. Additional details are available on the simeprevir sodium profile page.
When can XALKORI (crizotinib) generic drug versions launch?
Generic name: crizotinib
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: December 04, 2026
Generic Entry Controlled by: Argentina Patent 57,964
XALKORI is a drug marketed by Pf Prism Cv. There are five patents protecting this drug.
This drug has one hundred and fifty-two patent family members in forty-eight countries.
See drug price trends for XALKORI.
The generic ingredient in XALKORI is crizotinib. One supplier is listed for this generic product. Additional details are available on the crizotinib profile page.
When can BYDUREON (exenatide synthetic) generic drug versions launch?
Generic name: exenatide synthetic
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 28, 2026
Generic Entry Controlled by: Argentina Patent 61,730

This drug has three hundred and forty-six patent family members in forty-eight countries. There has been litigation on patents covering BYDUREON
See drug price trends for BYDUREON.
The generic ingredient in BYDUREON is exenatide synthetic. There are seven drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the exenatide synthetic profile page.
When can EPIDUO (adapalene; benzoyl peroxide) generic drug versions launch?
Generic name: adapalene; benzoyl peroxide
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: July 13, 2027
Generic Entry Controlled by: Argentina Patent 61,989
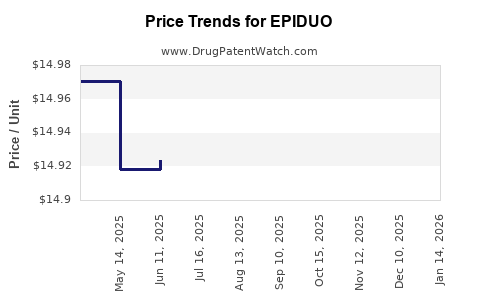
This drug has sixty-eight patent family members in twenty-five countries. There has been litigation on patents covering EPIDUO
See drug price trends for EPIDUO.
The generic ingredient in EPIDUO is adapalene; benzoyl peroxide. There are twelve drug master file entries for this API. Thirteen suppliers are listed for this generic product. Additional details are available on the adapalene; benzoyl peroxide profile page.
When can EPIDUO FORTE (adapalene; benzoyl peroxide) generic drug versions launch?
Generic name: adapalene; benzoyl peroxide
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: July 13, 2027
Generic Entry Controlled by: Argentina Patent 61,989
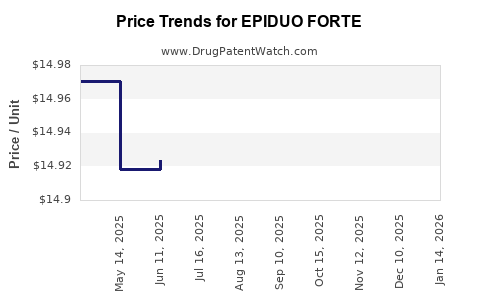
See drug price trends for EPIDUO FORTE.
The generic ingredient in EPIDUO FORTE is adapalene; benzoyl peroxide. There are twelve drug master file entries for this API. Thirteen suppliers are listed for this generic product. Additional details are available on the adapalene; benzoyl peroxide profile page.
When can INTRAROSA (prasterone) generic drug versions launch?
Generic name: prasterone
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 10, 2027
Generic Entry Controlled by: Argentina Patent 68,702
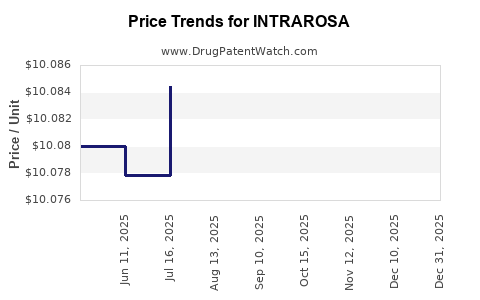
This drug has fifty-nine patent family members in thirty-two countries.
See drug price trends for INTRAROSA.
The generic ingredient in INTRAROSA is prasterone. There are seven drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the prasterone profile page.
When can LASTACAFT (alcaftadine) generic drug versions launch?
Generic name: alcaftadine
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: March 31, 2026
Generic Entry Controlled by: Argentina Patent 60,278
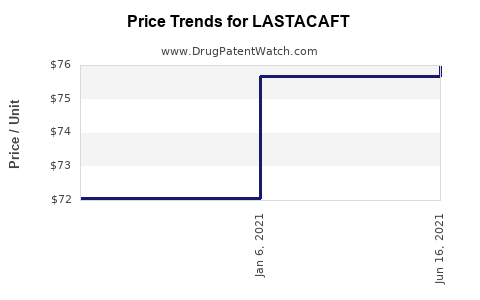
This drug has forty-six patent family members in thirty countries. There has been litigation on patents covering LASTACAFT
See drug price trends for LASTACAFT.
The generic ingredient in LASTACAFT is alcaftadine. There are six drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the alcaftadine profile page.
When can INVOKAMET XR (canagliflozin; metformin hydrochloride) generic drug versions launch?
Generic name: canagliflozin; metformin hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: December 03, 2027
Generic Entry Controlled by: Argentina Patent 64,099
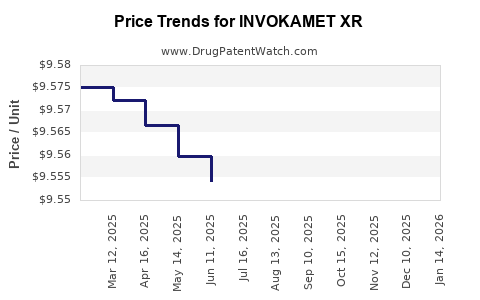
This drug has two hundred and twenty patent family members in forty-five countries. There has been litigation on patents covering INVOKAMET XR
See drug price trends for INVOKAMET XR.
The generic ingredient in INVOKAMET XR is canagliflozin; metformin hydrochloride. There are twenty-one drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the canagliflozin; metformin hydrochloride profile page.
When can APTIOM (eslicarbazepine acetate) generic drug versions launch?
Generic name: eslicarbazepine acetate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: April 24, 2026
Generic Entry Controlled by: Argentina Patent 55,917
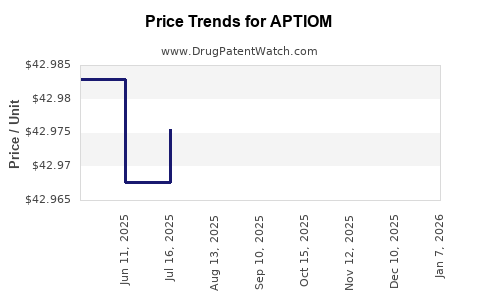
This drug has one hundred patent family members in twenty-six countries. There has been litigation on patents covering APTIOM
See drug price trends for APTIOM.
The generic ingredient in APTIOM is eslicarbazepine acetate. There are twelve drug master file entries for this API. Eight suppliers are listed for this generic product. Additional details are available on the eslicarbazepine acetate profile page.
When can BELSOMRA (suvorexant) generic drug versions launch?
Generic name: suvorexant
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: November 27, 2027
Generic Entry Controlled by: Argentina Patent 63,979
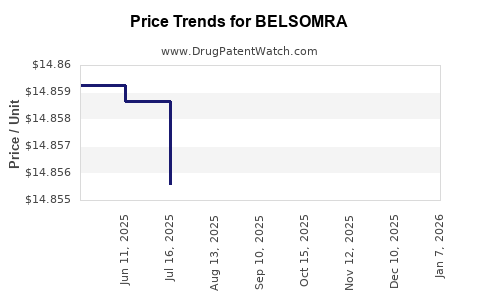
This drug has seventy-five patent family members in thirty-six countries.
See drug price trends for BELSOMRA.
The generic ingredient in BELSOMRA is suvorexant. One supplier is listed for this generic product. Additional details are available on the suvorexant profile page.
When can BOSULIF (bosutinib monohydrate) generic drug versions launch?
Generic name: bosutinib monohydrate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 29, 2026
Generic Entry Controlled by: Argentina Patent 54,505
BOSULIF is a drug marketed by Pf Prism Cv. There are four patents protecting this drug and two Paragraph IV challenges. Two tentatively approved generics are ready to enter the market.
This drug has eighty-one patent family members in thirty countries. There has been litigation on patents covering BOSULIF
See drug price trends for BOSULIF.
The generic ingredient in BOSULIF is bosutinib monohydrate. There are five drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the bosutinib monohydrate profile page.
When can BRILINTA (ticagrelor) generic drug versions launch?
Generic name: ticagrelor
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 15, 2027
Generic Entry Controlled by: Argentina Patent 62,451
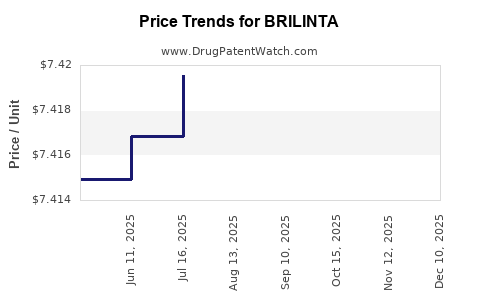
This drug has one hundred and forty-seven patent family members in forty-four countries. There has been litigation on patents covering BRILINTA
See drug price trends for BRILINTA.
The generic ingredient in BRILINTA is ticagrelor. There are twenty-one drug master file entries for this API. Twenty-five suppliers are listed for this generic product. Additional details are available on the ticagrelor profile page.
When can EXFORGE HCT (amlodipine besylate; hydrochlorothiazide; valsartan) generic drug versions launch?
Generic name: amlodipine besylate; hydrochlorothiazide; valsartan
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 27, 2026
Generic Entry Controlled by: Argentina Patent 61,627

See drug price trends for EXFORGE HCT.
The generic ingredient in EXFORGE HCT is amlodipine besylate; hydrochlorothiazide; valsartan. There are fifty drug master file entries for this API. Five suppliers are listed for this generic product. Additional details are available on the amlodipine besylate; hydrochlorothiazide; valsartan profile page.
When can FARXIGA (dapagliflozin) generic drug versions launch?
Generic name: dapagliflozin
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 28, 2026
Generic Entry Controlled by: Argentina Patent 61,730
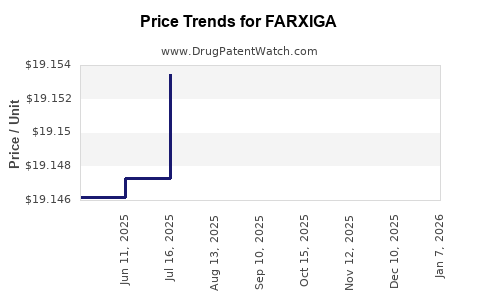
This drug has four hundred and fifty-one patent family members in fifty-two countries. There has been litigation on patents covering FARXIGA
See drug price trends for FARXIGA.
The generic ingredient in FARXIGA is dapagliflozin. There are twenty-six drug master file entries for this API. Five suppliers are listed for this generic product. Additional details are available on the dapagliflozin profile page.
When can GLYXAMBI (empagliflozin; linagliptin) generic drug versions launch?
Generic name: empagliflozin; linagliptin
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 16, 2027
Generic Entry Controlled by: Argentina Patent 87,657
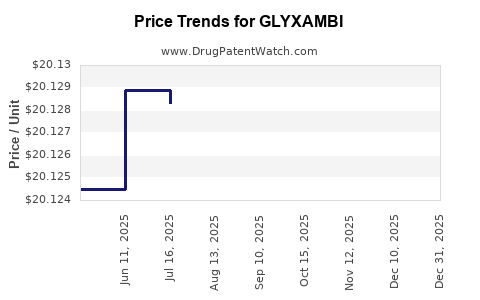
This drug has six hundred and twenty-six patent family members in forty-eight countries. There has been litigation on patents covering GLYXAMBI
See drug price trends for GLYXAMBI.
The generic ingredient in GLYXAMBI is empagliflozin; linagliptin. There are twenty-two drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the empagliflozin; linagliptin profile page.
When can INVOKAMET (canagliflozin; metformin hydrochloride) generic drug versions launch?
Generic name: canagliflozin; metformin hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: December 03, 2027
Generic Entry Controlled by: Argentina Patent 64,099
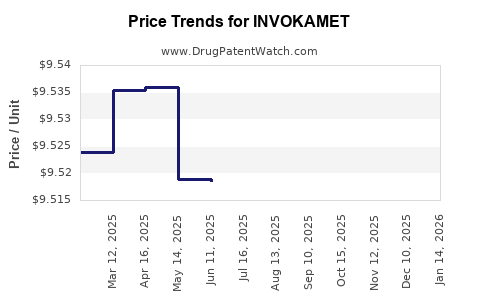
This drug has two hundred and seventy-one patent family members in forty-eight countries. There has been litigation on patents covering INVOKAMET
See drug price trends for INVOKAMET.
The generic ingredient in INVOKAMET is canagliflozin; metformin hydrochloride. There are twenty-one drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the canagliflozin; metformin hydrochloride profile page.
When can JARDIANCE (empagliflozin) generic drug versions launch?
Generic name: empagliflozin
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 16, 2027
Generic Entry Controlled by: Argentina Patent 87,657

This drug has three hundred and seventy-eight patent family members in forty-six countries. There has been litigation on patents covering JARDIANCE
See drug price trends for JARDIANCE.
The generic ingredient in JARDIANCE is empagliflozin. There are twenty-two drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the empagliflozin profile page.
When can OPSUMIT (macitentan) generic drug versions launch?
Generic name: macitentan
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 23, 2027
Generic Entry Controlled by: Argentina Patent 62,501
OPSUMIT is a drug marketed by Actelion. There are five patents protecting this drug and one Paragraph IV challenge. One tentatively approved generic is ready to enter the market.
This drug has ninety-nine patent family members in thirty-four countries. There has been litigation on patents covering OPSUMIT
See drug price trends for OPSUMIT.
The generic ingredient in OPSUMIT is macitentan. There are ten drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the macitentan profile page.
When can PROMACTA (eltrombopag olamine) generic drug versions launch?
Generic name: eltrombopag olamine
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 01, 2027
Generic Entry Controlled by: Argentina Patent 59,656
PROMACTA is a drug marketed by Novartis. There are six patents protecting this drug and two Paragraph IV challenges. One tentatively approved generic is ready to enter the market.
This drug has one hundred and thirty-four patent family members in forty-one countries. There has been litigation on patents covering PROMACTA
See drug price trends for PROMACTA.
The generic ingredient in PROMACTA is eltrombopag olamine. There are three drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the eltrombopag olamine profile page.
When can QTERN (dapagliflozin; saxagliptin hydrochloride) generic drug versions launch?
Generic name: dapagliflozin; saxagliptin hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 28, 2026
Generic Entry Controlled by: Argentina Patent 61,730
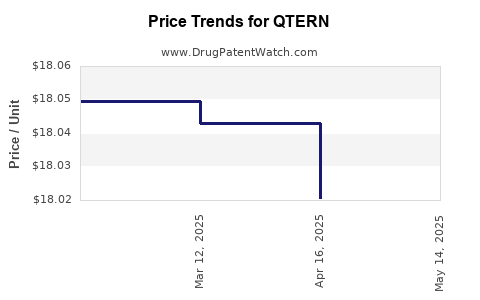
This drug has three hundred and thirteen patent family members in forty-eight countries. There has been litigation on patents covering QTERN
See drug price trends for QTERN.
The generic ingredient in QTERN is dapagliflozin; saxagliptin hydrochloride. There are twenty-six drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the dapagliflozin; saxagliptin hydrochloride profile page.
When can SIRTURO (bedaquiline fumarate) generic drug versions launch?
Generic name: bedaquiline fumarate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: December 05, 2027
Generic Entry Controlled by: Argentina Patent 64,149
SIRTURO is a drug marketed by Janssen Therap. There are two patents protecting this drug.
This drug has ninety-seven patent family members in thirty-nine countries.
See drug price trends for SIRTURO.
The generic ingredient in SIRTURO is bedaquiline fumarate. There is one drug master file entry for this API. One supplier is listed for this generic product. Additional details are available on the bedaquiline fumarate profile page.
When can TEKTURNA HCT (aliskiren hemifumarate; hydrochlorothiazide) generic drug versions launch?
Generic name: aliskiren hemifumarate; hydrochlorothiazide
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 23, 2026
Generic Entry Controlled by: Argentina Patent 61,565

This drug has thirty-two patent family members in twenty-five countries. There has been litigation on patents covering TEKTURNA HCT
See drug price trends for TEKTURNA HCT.
The generic ingredient in TEKTURNA HCT is aliskiren hemifumarate; hydrochlorothiazide. There are four drug master file entries for this API. Additional details are available on the aliskiren hemifumarate; hydrochlorothiazide profile page.
When can TRADJENTA (linagliptin) generic drug versions launch?
Generic name: linagliptin
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: May 04, 2026
Generic Entry Controlled by: Argentina Patent 60,755
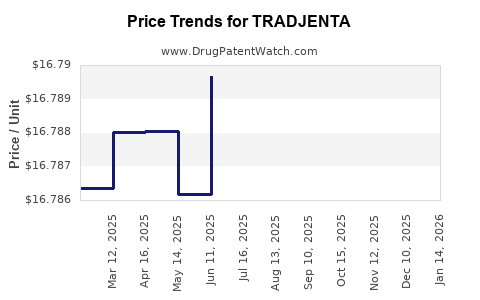
This drug has four hundred and eighty-six patent family members in forty-five countries. There has been litigation on patents covering TRADJENTA
See drug price trends for TRADJENTA.
The generic ingredient in TRADJENTA is linagliptin. There are nineteen drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the linagliptin profile page.
When can TRADJENTA (linagliptin) generic drug versions launch?
Generic name: linagliptin
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: May 04, 2026
Generic Entry Controlled by: Argentina Patent 79,930

This drug has four hundred and eighty-six patent family members in forty-five countries. There has been litigation on patents covering TRADJENTA
See drug price trends for TRADJENTA.
The generic ingredient in TRADJENTA is linagliptin. There are nineteen drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the linagliptin profile page.
When can TRINTELLIX (vortioxetine hydrobromide) generic drug versions launch?
Generic name: vortioxetine hydrobromide
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 16, 2026
Generic Entry Controlled by: Argentina Patent 61,481
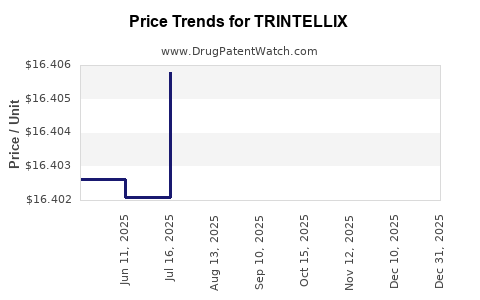
This drug has two hundred and seventeen patent family members in forty-two countries. There has been litigation on patents covering TRINTELLIX
See drug price trends for TRINTELLIX.
The generic ingredient in TRINTELLIX is vortioxetine hydrobromide. There are sixteen drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the vortioxetine hydrobromide profile page.
When can VARUBI (rolapitant hydrochloride) generic drug versions launch?
Generic name: rolapitant hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: March 22, 2027
Generic Entry Controlled by: Argentina Patent 65,802
VARUBI is a drug marketed by Tersera. There are eight patents protecting this drug.
This drug has one hundred and fifty-eight patent family members in thirty-five countries.
The generic ingredient in VARUBI is rolapitant hydrochloride. One supplier is listed for this generic product. Additional details are available on the rolapitant hydrochloride profile page.
When can XADAGO (safinamide mesylate) generic drug versions launch?
Generic name: safinamide mesylate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: June 19, 2027
Generic Entry Controlled by: Argentina Patent 61,510
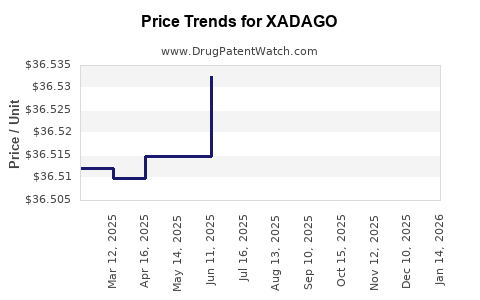
This drug has ninety-seven patent family members in thirty-one countries. There has been litigation on patents covering XADAGO
See drug price trends for XADAGO.
The generic ingredient in XADAGO is safinamide mesylate. There are two drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the safinamide mesylate profile page.
When can WINLEVI (clascoterone) generic drug versions launch?
Generic name: clascoterone
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 03, 2027
Generic Entry Controlled by: Argentina Patent 111,351

This drug has ninety patent family members in twenty-seven countries. There has been litigation on patents covering WINLEVI
See drug price trends for WINLEVI.
The generic ingredient in WINLEVI is clascoterone. One supplier is listed for this generic product. Additional details are available on the clascoterone profile page.
When can XERMELO (telotristat etiprate) generic drug versions launch?
Generic name: telotristat etiprate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: December 11, 2027
Generic Entry Controlled by: Argentina Patent 64,279
XERMELO is a drug marketed by Tersera. There are five patents protecting this drug.
This drug has seventy patent family members in twenty-nine countries.
See drug price trends for XERMELO.
The generic ingredient in XERMELO is telotristat etiprate. One supplier is listed for this generic product. Additional details are available on the telotristat etiprate profile page.
When can AMELUZ (aminolevulinic acid hydrochloride) generic drug versions launch?
Generic name: aminolevulinic acid hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: December 22, 2026
Generic Entry Controlled by: Argentina Patent 64,659
AMELUZ is a drug marketed by Biofrontera. There are three patents protecting this drug.
This drug has twenty-nine patent family members in eighteen countries.
See drug price trends for AMELUZ.
The generic ingredient in AMELUZ is aminolevulinic acid hydrochloride. There are six drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the aminolevulinic acid hydrochloride profile page.
When can INPEFA (sotagliflozin) generic drug versions launch?
Generic name: sotagliflozin
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: September 28, 2027
Generic Entry Controlled by: Argentina Patent 63,047
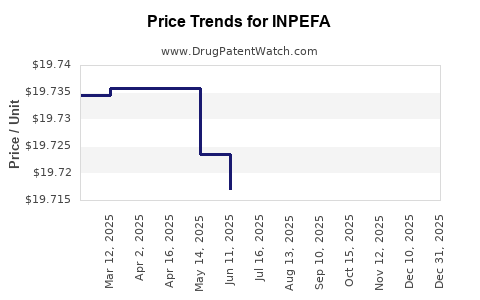
This drug has eighty-one patent family members in thirty-three countries.
See drug price trends for INPEFA.
The generic ingredient in INPEFA is sotagliflozin. Additional details are available on the sotagliflozin profile page.
When can ENTRESTO SPRINKLE (sacubitril; valsartan) generic drug versions launch?
Generic name: sacubitril; valsartan
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: November 07, 2026
Generic Entry Controlled by: Argentina Patent 57,882
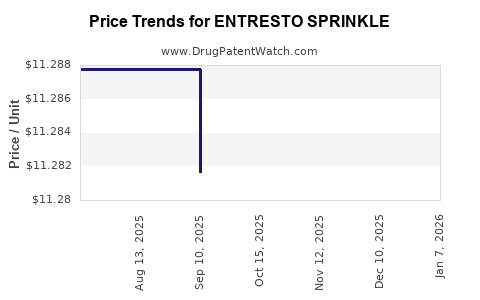
This drug has one hundred and forty-five patent family members in forty-three countries. There has been litigation on patents covering ENTRESTO SPRINKLE
See drug price trends for ENTRESTO SPRINKLE.
The generic ingredient in ENTRESTO SPRINKLE is sacubitril; valsartan. There are eleven drug master file entries for this API. Twenty-two suppliers are listed for this generic product. Additional details are available on the sacubitril; valsartan profile page.
When can OPSYNVI (macitentan; tadalafil) generic drug versions launch?
Generic name: macitentan; tadalafil
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: August 23, 2027
Generic Entry Controlled by: Argentina Patent 62,501
OPSYNVI is a drug marketed by Actelion. There are three patents protecting this drug.
This drug has ninety-nine patent family members in thirty-four countries. There has been litigation on patents covering OPSYNVI
See drug price trends for OPSYNVI.
The generic ingredient in OPSYNVI is macitentan; tadalafil. There are ten drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the macitentan; tadalafil profile page.
When can INVOKANA (canagliflozin) generic drug versions launch?
Generic name: canagliflozin
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: December 03, 2027
Generic Entry Controlled by: Argentina Patent 64,099
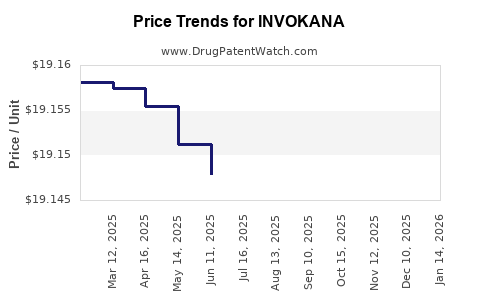
This drug has two hundred and twenty patent family members in forty-five countries. There has been litigation on patents covering INVOKANA
See drug price trends for INVOKANA.
The generic ingredient in INVOKANA is canagliflozin. There are twenty-one drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the canagliflozin profile page.
When can REVLIMID (lenalidomide) generic drug versions launch?
Generic name: lenalidomide
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: October 03, 2026
Generic Entry Controlled by: Argentina Patent 57,868
REVLIMID is a drug marketed by Bristol Myers Squibb. There are two patents protecting this drug and three Paragraph IV challenges.
This drug has three hundred and thirty-one patent family members in forty-one countries. There has been litigation on patents covering REVLIMID
See drug price trends for REVLIMID.
The generic ingredient in REVLIMID is lenalidomide. There are fourteen drug master file entries for this API. Fifteen suppliers are listed for this generic product. Additional details are available on the lenalidomide profile page.
When can VICTRELIS (boceprevir) generic drug versions launch?
Generic name: boceprevir
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: May 31, 2026
Generic Entry Controlled by: Argentina Patent 55,198
VICTRELIS is a drug marketed by Merck Sharp Dohme. There are two patents protecting this drug.
This drug has twenty-seven patent family members in seventeen countries.
The generic ingredient in VICTRELIS is boceprevir. Additional details are available on the boceprevir profile page.
When can SELZENTRY (maraviroc) generic drug versions launch?
Generic name: maraviroc
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: March 27, 2027
Generic Entry Controlled by: Argentina Patent 60,159
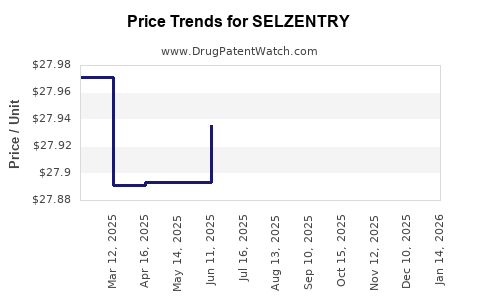
See drug price trends for SELZENTRY.
The generic ingredient in SELZENTRY is maraviroc. There are two drug master file entries for this API. Nine suppliers are listed for this generic product. Additional details are available on the maraviroc profile page.
When can VIMOVO (esomeprazole magnesium; naproxen) generic drug versions launch?
Generic name: esomeprazole magnesium; naproxen
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 20, 2027
Generic Entry Controlled by: Argentina Patent 59,589
VIMOVO is a drug marketed by Horizon. One tentatively approved generic is ready to enter the market. There has been litigation on patents covering VIMOVO
See drug price trends for VIMOVO.
The generic ingredient in VIMOVO is esomeprazole magnesium; naproxen. There are seventy-four drug master file entries for this API. Eight suppliers are listed for this generic product. Additional details are available on the esomeprazole magnesium; naproxen profile page.
When can ACTOPLUS MET XR (metformin hydrochloride; pioglitazone hydrochloride) generic drug versions launch?
Generic name: metformin hydrochloride; pioglitazone hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: March 15, 2026
Generic Entry Controlled by: Argentina Patent 54,238

This drug has seventy-two patent family members in twenty-five countries. There has been litigation on patents covering ACTOPLUS MET XR
See drug price trends for ACTOPLUS MET XR.
The generic ingredient in ACTOPLUS MET XR is metformin hydrochloride; pioglitazone hydrochloride. There are forty-nine drug master file entries for this API. Six suppliers are listed for this generic product. Additional details are available on the metformin hydrochloride; pioglitazone hydrochloride profile page.
When can AVANDAMET (metformin hydrochloride; rosiglitazone maleate) generic drug versions launch?
Generic name: metformin hydrochloride; rosiglitazone maleate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: December 05, 2026
Generic Entry Controlled by: Argentina Patent 57,970
AVANDAMET is a drug marketed by Sb Pharmco. There has been litigation on patents covering AVANDAMET
The generic ingredient in AVANDAMET is metformin hydrochloride; rosiglitazone maleate. There are forty-nine drug master file entries for this API. Additional details are available on the metformin hydrochloride; rosiglitazone maleate profile page.
When can CORLANOR (ivabradine hydrochloride) generic drug versions launch?
Generic name: ivabradine hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 28, 2026
Generic Entry Controlled by: Argentina Patent 53,147
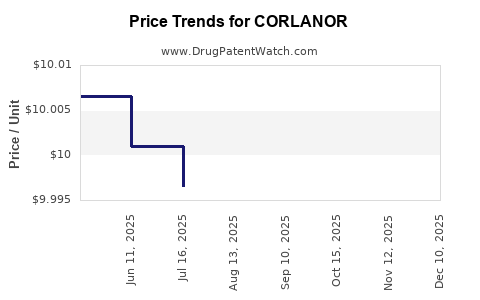
This drug has ninety-seven patent family members in forty-two countries. There has been litigation on patents covering CORLANOR
See drug price trends for CORLANOR.
The generic ingredient in CORLANOR is ivabradine hydrochloride. There are nine drug master file entries for this API. Eleven suppliers are listed for this generic product. Additional details are available on the ivabradine hydrochloride profile page.
When can EPZICOM (abacavir sulfate; lamivudine) generic drug versions launch?
Generic name: abacavir sulfate; lamivudine
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: January 02, 2027
Generic Entry Controlled by: Argentina Patent 59,120
EPZICOM is a drug marketed by Viiv Hlthcare. Five tentatively approved generics are ready to enter the market. There has been litigation on patents covering EPZICOM
See drug price trends for EPZICOM.
The generic ingredient in EPZICOM is abacavir sulfate; lamivudine. There are twelve drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the abacavir sulfate; lamivudine profile page.
When can NEXAVAR (sorafenib tosylate) generic drug versions launch?
Generic name: sorafenib tosylate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: March 03, 2026
Generic Entry Controlled by: Argentina Patent 54,234
NEXAVAR is a drug marketed by Bayer Hlthcare. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has eighty-nine patent family members in thirty-nine countries. There has been litigation on patents covering NEXAVAR
See drug price trends for NEXAVAR.
The generic ingredient in NEXAVAR is sorafenib tosylate. There are thirteen drug master file entries for this API. Eight suppliers are listed for this generic product. Additional details are available on the sorafenib tosylate profile page.
When can TRIZIVIR (abacavir sulfate; lamivudine; zidovudine) generic drug versions launch?
Generic name: abacavir sulfate; lamivudine; zidovudine
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: January 02, 2027
Generic Entry Controlled by: Argentina Patent 59,120
TRIZIVIR is a drug marketed by Viiv Hlthcare. One tentatively approved generic is ready to enter the market. There has been litigation on patents covering TRIZIVIR
See drug price trends for TRIZIVIR.
The generic ingredient in TRIZIVIR is abacavir sulfate; lamivudine; zidovudine. There are twelve drug master file entries for this API. Additional details are available on the abacavir sulfate; lamivudine; zidovudine profile page.
When can TYKERB (lapatinib ditosylate) generic drug versions launch?
Generic name: lapatinib ditosylate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: April 18, 2026
Generic Entry Controlled by: Argentina Patent 54,252
TYKERB is a drug marketed by Novartis. There is one patent protecting this drug and one Paragraph IV challenge.
This drug has twenty-eight patent family members in twenty-six countries.
See drug price trends for TYKERB.
The generic ingredient in TYKERB is lapatinib ditosylate. There are seven drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the lapatinib ditosylate profile page.
When can CORLANOR (ivabradine) generic drug versions launch?
Generic name: ivabradine
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 28, 2026
Generic Entry Controlled by: Argentina Patent 52,926

This drug has ninety-seven patent family members in forty-two countries. There has been litigation on patents covering CORLANOR
See drug price trends for CORLANOR.
The generic ingredient in CORLANOR is ivabradine. There are nine drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the ivabradine profile page.
When can WAKIX (pitolisant hydrochloride) generic drug versions launch?
Generic name: pitolisant hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: February 08, 2026
Generic Entry Controlled by: Argentina Patent 54,734
WAKIX is a drug marketed by Harmony. There are three patents protecting this drug.
This drug has sixty-one patent family members in thirty-one countries. There has been litigation on patents covering WAKIX
See drug price trends for WAKIX.
The generic ingredient in WAKIX is pitolisant hydrochloride. One supplier is listed for this generic product. Additional details are available on the pitolisant hydrochloride profile page.
When can XCOPRI (cenobamate) generic drug versions launch?
Generic name: cenobamate
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: April 21, 2026
Generic Entry Controlled by: Argentina Patent 53,065
This drug has twenty-six patent family members in twenty countries. There has been litigation on patents covering XCOPRI
See drug price trends for XCOPRI.
The generic ingredient in XCOPRI is cenobamate. One supplier is listed for this generic product. Additional details are available on the cenobamate profile page.
When can BELRAPZO (bendamustine hydrochloride) generic drug versions launch?
Generic name: bendamustine hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: January 13, 2026
Generic Entry Controlled by: Argentina Patent 52,877
BELRAPZO is a drug marketed by Eagle Pharms. There are eleven patents protecting this drug. Six tentatively approved generics are ready to enter the market.
This drug has sixty-five patent family members in thirty countries. There has been litigation on patents covering BELRAPZO
See drug price trends for BELRAPZO.
The generic ingredient in BELRAPZO is bendamustine hydrochloride. There are twenty-three drug master file entries for this API. Eleven suppliers are listed for this generic product. Additional details are available on the bendamustine hydrochloride profile page.
When can BENDEKA (bendamustine hydrochloride) generic drug versions launch?
Generic name: bendamustine hydrochloride
DrugPatentWatch® Estimated Patent Expiration / Generic Entry Opportunity Date: January 13, 2026
Generic Entry Controlled by: Argentina Patent 52,877
BENDEKA is a drug marketed by Eagle Pharms. There are twenty patents protecting this drug and one Paragraph IV challenge. Six tentatively approved generics are ready to enter the market.
This drug has one hundred and twenty-five patent family members in thirty-one countries. There has been litigation on patents covering BENDEKA
See drug price trends for BENDEKA.
The generic ingredient in BENDEKA is bendamustine hydrochloride. There are twenty-three drug master file entries for this API. Eleven suppliers are listed for this generic product. Additional details are available on the bendamustine hydrochloride profile page.
DrugPatentWatch cited by CNN, NEJM, Nature Journals, and more …

Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
