AMYVID Drug Patent Profile
✉ Email this page to a colleague
When do Amyvid patents expire, and what generic alternatives are available?
Amyvid is a drug marketed by Avid Radiopharms Inc and is included in one NDA. There are two patents protecting this drug.
This drug has fifty-one patent family members in thirty-three countries.
The generic ingredient in AMYVID is florbetapir f-18. One supplier is listed for this compound. Additional details are available on the florbetapir f-18 profile page.
DrugPatentWatch® Generic Entry Outlook for Amyvid
Amyvid was eligible for patent challenges on April 6, 2016.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be April 30, 2027. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for AMYVID?
- What are the global sales for AMYVID?
- What is Average Wholesale Price for AMYVID?
Summary for AMYVID
| International Patents: | 51 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 40 |
| Patent Applications: | 391 |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for AMYVID |
| What excipients (inactive ingredients) are in AMYVID? | AMYVID excipients list |
| DailyMed Link: | AMYVID at DailyMed |
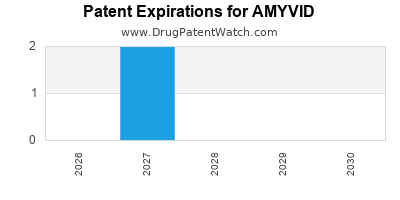
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for AMYVID
Generic Entry Date for AMYVID*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for AMYVID
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Columbia University | PHASE1 |
| Universidad Central del Caribe | Phase 2 |
| University of Puerto Rico | Phase 2 |
Pharmacology for AMYVID
| Drug Class | Radioactive Diagnostic Agent |
| Mechanism of Action | Positron Emitting Activity |
US Patents and Regulatory Information for AMYVID
AMYVID is protected by two US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of AMYVID is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Avid Radiopharms Inc | AMYVID | florbetapir f-18 | SOLUTION;INTRAVENOUS | 202008-001 | Apr 6, 2012 | DISCN | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Avid Radiopharms Inc | AMYVID | florbetapir f-18 | SOLUTION;INTRAVENOUS | 202008-003 | Apr 6, 2012 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Avid Radiopharms Inc | AMYVID | florbetapir f-18 | SOLUTION;INTRAVENOUS | 202008-004 | Oct 13, 2023 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Avid Radiopharms Inc | AMYVID | florbetapir f-18 | SOLUTION;INTRAVENOUS | 202008-003 | Apr 6, 2012 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for AMYVID
When does loss-of-exclusivity occur for AMYVID?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 07243712
Estimated Expiration: ⤷ Get Started Free
Austria
Patent: 39060
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0710225
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 44530
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1522624
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 329
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0120135
Estimated Expiration: ⤷ Get Started Free
Patent: 0170857
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 13048
Estimated Expiration: ⤷ Get Started Free
Patent: 19048
Estimated Expiration: ⤷ Get Started Free
Patent: 13024
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 99109
Estimated Expiration: ⤷ Get Started Free
Patent: 63392
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 088783
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 7898
Estimated Expiration: ⤷ Get Started Free
Patent: 0870389
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 99109
Estimated Expiration: ⤷ Get Started Free
Patent: 63391
Estimated Expiration: ⤷ Get Started Free
Patent: 63392
Estimated Expiration: ⤷ Get Started Free
France
Patent: C0034
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 0800201
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 32660
Estimated Expiration: ⤷ Get Started Free
Patent: 300028
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 3567
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 90954
Estimated Expiration: ⤷ Get Started Free
Patent: 09532349
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 63392
Estimated Expiration: ⤷ Get Started Free
Luxembourg
Patent: 232
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 08012527
Patent: DERIVADOS DE ESTIRILPIRIDINA Y SUS USOS PARA UNION A PLACAS AMILOIDES Y OBTENCION DE IMAGENES DE LAS MISMAS. (STYRYLPYRIDINE DERIVATIVES AND THEIR USE FOR BINDING AND IMAGING AMYLOID PLAQUES.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 0887
Patent: Styrylpyridine derivatives and their use for binding and imaging amyloid plaques
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 2090
Estimated Expiration: ⤷ Get Started Free
Patent: 18030
Estimated Expiration: ⤷ Get Started Free
Patent: 084590
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 99109
Estimated Expiration: ⤷ Get Started Free
Patent: 63392
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 99109
Estimated Expiration: ⤷ Get Started Free
Patent: 63392
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 222
Patent: DERIVATI STIRILPIRIDINA I NJIHOVA UPOTREBA ZA VEZIVANJE I IMIDŽING AMILOIDNIH PLAKOVA (STYRYLPYRIDINE DERIVATIVES AND THEIR USE FOR BINDING AND IMAGING AMYLOID PLAQUES)
Estimated Expiration: ⤷ Get Started Free
Patent: 171
Patent: DERIVATI STIRILPIRIDINA I NJIHOVA UPOTREBA ZA VEZIVANJE I IMIDŽING AMILOIDNIH PLAKOVA (STYRYLPYRIDINE DERIVATIVES AND THEIR USE FOR BINDING AND IMAGING AMYLOID PLAQUES)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 3338
Patent: STYRYLPYRIDINE DERIVATIVES AND THEIR USE FOR BINDING AND IMAGING AMYLOID PLAQUES
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 99109
Estimated Expiration: ⤷ Get Started Free
Patent: 63392
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0807955
Patent: STRYLPYRIDINE DERIVATIVES AND THEIR USE FOR BINDING AND IMAGING AMYLOID PLAQUES
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1376807
Estimated Expiration: ⤷ Get Started Free
Patent: 080106564
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 78785
Estimated Expiration: ⤷ Get Started Free
Patent: 28882
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 99366
Estimated Expiration: ⤷ Get Started Free
Patent: 0838852
Patent: Styrylpyridine derivatives and their use for binding and imaging amyloid plaques
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 802
Patent: СТИРИЛПИРИДИНОВЫЕ ПРОИЗВОДНЫЕ И ИХ ПРИМЕНЕНИЕ ДЛЯ СВЯЗЫВАНИЯ И ВИЗУАЛИЗАЦИИ АМИЛОИДНЫХ БЛЯШЕК;СТИРИЛПІРИДИНОВІ ПОХІДНІ І ЇХ ЗАСТОСУВАННЯ ДЛЯ ЗВ'ЯЗУВАННЯ І ВІЗУАЛІЗАЦІЇ АМІЛОЇДНИХ БЛЯШОК (STYRYLPYRIDINE COMPOUNDS USEFUL AND USE THEREOF IN IMAGING AMYLOID DEPOSITS)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering AMYVID around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Hungary | E032660 | ⤷ Get Started Free | |
| Portugal | 1999109 | ⤷ Get Started Free | |
| Norway | 342090 | ⤷ Get Started Free | |
| Portugal | 2363392 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for AMYVID
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1999109 | C300600 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: FLORBETAPIR ( SUP 18 /SUP F); REGISTRATION NO/DATE: EU/1/12/805 20130114 |
| 1999109 | 139 5017-2013 | Slovakia | ⤷ Get Started Free | PRODUCT NAME: FLORBETAPIR ( 18 F); REGISTRATION NO/DATE: EU/1/12/805 20130114 |
| 1999109 | 92232 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: FLORBETAPIR (18 F) |
| 1999109 | 122013000051 | Germany | ⤷ Get Started Free | PRODUCT NAME: FLORBETAPIR (18F); REGISTRATION NO/DATE: EU/1/12/805 20130114 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for AMYVID
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
