Abbvie Company Profile
✉ Email this page to a colleague
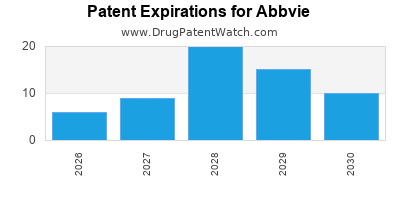
What is the competitive landscape for ABBVIE, and when can generic versions of ABBVIE drugs launch?
ABBVIE has one hundred and thirty-eight approved drugs.
There are two hundred and twenty-nine US patents protecting ABBVIE drugs.
There are two thousand seven hundred and twenty-six patent family members on ABBVIE drugs in sixty-six countries and three hundred and ninety-three supplementary protection certificates in nineteen countries.
Summary for Abbvie
| International Patents: | 2726 |
| US Patents: | 229 |
| Tradenames: | 124 |
| Ingredients: | 99 |
| NDAs: | 138 |
| Drug Master File Entries: | 1 |
| Patent Litigation for Abbvie: | See patent lawsuits for Abbvie |
Drugs and US Patents for Abbvie
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbvie | LINZESS | linaclotide | CAPSULE;ORAL | 202811-001 | Aug 30, 2012 | RX | Yes | Yes | 8,933,030 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Abbvie | VIEKIRA XR | dasabuvir sodium; ombitasvir; paritaprevir; ritonavir | TABLET, EXTENDED RELEASE;ORAL | 208624-001 | Jul 22, 2016 | DISCN | Yes | No | 10,201,541 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Abbvie | NAMZARIC | donepezil hydrochloride; memantine hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 206439-001 | Dec 23, 2014 | RX | Yes | No | 8,173,708*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Abbvie | ACULAR LS | ketorolac tromethamine | SOLUTION/DROPS;OPHTHALMIC | 021528-001 | May 30, 2003 | AT | RX | Yes | Yes | 8,906,950 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Abbvie | MAVYRET | glecaprevir; pibrentasvir | TABLET;ORAL | 209394-001 | Aug 3, 2017 | RX | Yes | Yes | RE48923*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Abbvie
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie Endocrine Inc | LUPRON DEPOT | leuprolide acetate | INJECTABLE;INJECTION | 020517-002 | May 30, 1997 | 5,476,663 | ⤷ Try a Trial |
| Abbvie | SAVELLA | milnacipran hydrochloride | TABLET;ORAL | 022256-003 | Jan 14, 2009 | 6,602,911 | ⤷ Try a Trial |
| Abbvie | ACULAR | ketorolac tromethamine | SOLUTION/DROPS;OPHTHALMIC | 019700-001 | Nov 9, 1992 | 5,110,493*PED | ⤷ Try a Trial |
| Abbvie | KALETRA | lopinavir; ritonavir | SOLUTION;ORAL | 021251-001 | Sep 15, 2000 | 5,484,801*PED | ⤷ Try a Trial |
| Abbvie | ALPHAGAN P | brimonidine tartrate | SOLUTION/DROPS;OPHTHALMIC | 021770-001 | Aug 19, 2005 | 6,641,834*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for ABBVIE drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Delayed-release Tablets | 800 mg | ➤ Subscribe | 2011-07-13 |
| ➤ Subscribe | Extended-release Capsules | 7 mg, 14 mg, 21 mg, and 28 mg | ➤ Subscribe | 2013-06-10 |
| ➤ Subscribe | Ophthalmic Solution | 0.40% | ➤ Subscribe | 2005-01-28 |
| ➤ Subscribe | Tablets | 10 mg, 20 mg, and 40 mg | ➤ Subscribe | 2015-01-21 |
| ➤ Subscribe | Extended-release Tablets | 250 mg | ➤ Subscribe | 2004-05-03 |
| ➤ Subscribe | Capsules | 21 mg/10 mg | ➤ Subscribe | 2016-09-23 |
| ➤ Subscribe | Opthalmic Solution | 0.45% | ➤ Subscribe | 2011-08-24 |
| ➤ Subscribe | Extended-release Tablets | 7.5 mg and 15 mg | ➤ Subscribe | 2008-12-22 |
| ➤ Subscribe | Tablets | 100 mg/25 mg | ➤ Subscribe | 2008-12-23 |
| ➤ Subscribe | Ophthalmic Solution | 0.15% | ➤ Subscribe | 2006-11-03 |
| ➤ Subscribe | Capsules | 145 mcg and 290 mcg | ➤ Subscribe | 2016-08-30 |
| ➤ Subscribe | Extended-release Tablets | 1 mg/240 mg | ➤ Subscribe | 2008-02-20 |
| ➤ Subscribe | Ophthalmic Solution | 0.25% | ➤ Subscribe | 2014-07-30 |
| ➤ Subscribe | Capsules | 100 mg | ➤ Subscribe | 2012-10-31 |
| ➤ Subscribe | Tablets | 5 mg/80 mg | ➤ Subscribe | 2017-06-09 |
| ➤ Subscribe | Ophthalmic Solution | 0.01% | ➤ Subscribe | 2011-04-05 |
| ➤ Subscribe | Delayed-release Capsules | 400 mg | ➤ Subscribe | 2014-06-17 |
| ➤ Subscribe | Capsules | 1 mcg and 2 mcg | ➤ Subscribe | 2008-10-14 |
| ➤ Subscribe | Tablets | 5 mg and 10 mg | ➤ Subscribe | 2007-10-16 |
| ➤ Subscribe | Topical Solution | 0.03% | ➤ Subscribe | 2010-05-03 |
| ➤ Subscribe | Delayed-release Capsules | 135 mg | ➤ Subscribe | 2009-09-01 |
| ➤ Subscribe | Gel | 7.5% | ➤ Subscribe | 2017-02-13 |
| ➤ Subscribe | Tablets | 400 mg and 600 mg | ➤ Subscribe | 2010-05-10 |
| ➤ Subscribe | Capsules | 10 mg and 20 mg | ➤ Subscribe | 2005-03-30 |
| ➤ Subscribe | Extended-release Capsules | 7 mg, 14 mg, 21 mg, and 28 mg | ➤ Subscribe | 2013-08-16 |
| ➤ Subscribe | Injection | 2 mg/mL, 5 mL vial and 10 mg/mL, 20 mL vial | ➤ Subscribe | 2009-08-04 |
| ➤ Subscribe | Tablets | 145 mg | ➤ Subscribe | 2007-10-19 |
| ➤ Subscribe | Suppository | 1000 mg | ➤ Subscribe | 2013-05-24 |
| ➤ Subscribe | Extended-release Capsules | 7 mg, 14 mg, 21 mg | ➤ Subscribe | 2013-06-17 |
| ➤ Subscribe | Injection | 400 mg/vial and 600 mg/vial | ➤ Subscribe | 2014-10-29 |
| ➤ Subscribe | Tablets | 5 mg/200 mg | ➤ Subscribe | 2005-05-27 |
| ➤ Subscribe | Extended-release Tablet | 500 mg | ➤ Subscribe | 2010-12-06 |
| ➤ Subscribe | Capsules | 14 mg/10 mg and 28 mg/10 mg | ➤ Subscribe | 2015-05-18 |
| ➤ Subscribe | Opthalmic Solution | 0.45% | ➤ Subscribe | 2011-08-23 |
| ➤ Subscribe | Extended-release Tablets | 500 mg | ➤ Subscribe | 2005-02-08 |
| ➤ Subscribe | Capsules | 7 mg/10 mg | ➤ Subscribe | 2016-09-26 |
| ➤ Subscribe | Ophthalmic Solution | 0.10% | ➤ Subscribe | 2006-12-20 |
| ➤ Subscribe | Extended-release Tablets | 2 mg/180 mg and 2 mg/240 mg | ➤ Subscribe | 2007-11-09 |
| ➤ Subscribe | Oral Solution | 80 mg/20 mg per mL | ➤ Subscribe | 2014-06-19 |
| ➤ Subscribe | Ophthalmic Solution | 0.2%/0.5% | ➤ Subscribe | 2008-11-21 |
| ➤ Subscribe | Tablets | 100 mg | ➤ Subscribe | 2010-12-21 |
| ➤ Subscribe | Extended-release Tablets | 4 mg/ 240 mg | ➤ Subscribe | 2007-07-24 |
| ➤ Subscribe | Ophthalmic Solution | 0.03% | ➤ Subscribe | 2008-12-22 |
| ➤ Subscribe | Injection | 0.002 mg per mL in 1 mL vial and 0.005 mg per mL in 1 mL and 2 mL vials | ➤ Subscribe | 2008-11-28 |
| ➤ Subscribe | Tablets | 1 mg, 2 mg and 4 mg | ➤ Subscribe | 2004-10-04 |
| ➤ Subscribe | Ophthalmic Solution | 0.5% | ➤ Subscribe | 2010-12-07 |
| ➤ Subscribe | Delayed-release Capsules | 45 mg | ➤ Subscribe | 2009-09-02 |
| ➤ Subscribe | Capsules | 4 mcg | ➤ Subscribe | 2008-08-25 |
| ➤ Subscribe | Tablets | 12.5 mg, 25 mg, 50 mg, and 100 mg | ➤ Subscribe | 2013-01-14 |
| ➤ Subscribe | Capsules | 5 mg | ➤ Subscribe | 2005-08-17 |
| ➤ Subscribe | Extended-release Capsules | 20 mg, 40 mg, 80 mg and 120 mg | ➤ Subscribe | 2017-07-25 |
| ➤ Subscribe | Gel | 10% | ➤ Subscribe | 2014-06-19 |
| ➤ Subscribe | Tablets | 48 mg | ➤ Subscribe | 2008-07-01 |
| ➤ Subscribe | Injection | 10 mg/mL (2 mL) | ➤ Subscribe | 2018-07-13 |
| ➤ Subscribe | Extended-release Capsules | 28 mg | ➤ Subscribe | 2013-06-12 |
| ➤ Subscribe | Injection | 2 mg/mL, 10 mL vial | ➤ Subscribe | 2009-08-12 |
| ➤ Subscribe | Tablets | 2.5 mg/200 mg | ➤ Subscribe | 2006-02-24 |
International Patents for Abbvie Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Japan | 2019085415 | ⤷ Try a Trial |
| South Korea | 20030017500 | ⤷ Try a Trial |
| Brazil | 112016013690 | ⤷ Try a Trial |
| Japan | 2010168119 | ⤷ Try a Trial |
| China | 111437386 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Abbvie Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0403159 | C300006 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: EPROSARTAN, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER HET MESYLAAT; NAT. REGISTRATION NO/DATE: RVG 22258 - RVG 22260 19980106; FIRST REGISTRATION: DE 39573.00.00 - 39573.00.00 19970417 |
| 0334429 | SPC/GB96/048 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: NEBIVOLOL, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT OR HYDRATE; REGISTERED: NL RVG/19317 19951018; UK 00242/0309 19960509 |
| 2822954 | 1890030-8 | Sweden | ⤷ Try a Trial | PRODUCT NAME: BICTEGRAVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR BICTEGRAVIR SODIUM; REG. NO/DATE: EU/1/18/1289 20180625 |
| 1725537 | 122017000009 | Germany | ⤷ Try a Trial | PRODUCT NAME: ELUXADOLIN ODER EIN PHARMAZEUTISCH AKZEPTABLES SALZ DAVON; REGISTRATION NO/DATE: EU/1/16/1126 20160919 |
| 1758590 | 132017000066197 | Italy | ⤷ Try a Trial | PRODUCT NAME: SALE SODICO DI ACIDO DESOSSICOLICO(BELKYRA); AUTHORISATION NUMBER(S) AND DATE(S): 044896013, 20161219;IS/1/16/071/01, 20160729 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.