TEPMETKO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Tepmetko, and what generic alternatives are available?
Tepmetko is a drug marketed by Emd Serono Inc and is included in one NDA. There are eight patents protecting this drug.
This drug has seventy-nine patent family members in thirty-six countries.
The generic ingredient in TEPMETKO is tepotinib hydrochloride. One supplier is listed for this compound. Additional details are available on the tepotinib hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Tepmetko
Tepmetko was eligible for patent challenges on February 3, 2025.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 19, 2030. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TEPMETKO?
- What are the global sales for TEPMETKO?
- What is Average Wholesale Price for TEPMETKO?
Summary for TEPMETKO
| International Patents: | 79 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 8 |
| Patent Applications: | 147 |
| Drug Prices: | Drug price information for TEPMETKO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TEPMETKO |
| What excipients (inactive ingredients) are in TEPMETKO? | TEPMETKO excipients list |
| DailyMed Link: | TEPMETKO at DailyMed |
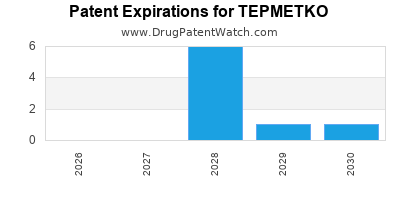

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TEPMETKO
Generic Entry Date for TEPMETKO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for TEPMETKO
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Mesenchymal Epithelial Transition Inhibitors P-Glycoprotein Inhibitors |
US Patents and Regulatory Information for TEPMETKO
TEPMETKO is protected by eight US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TEPMETKO is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Emd Serono Inc | TEPMETKO | tepotinib hydrochloride | TABLET;ORAL | 214096-001 | Feb 3, 2021 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Emd Serono Inc | TEPMETKO | tepotinib hydrochloride | TABLET;ORAL | 214096-001 | Feb 3, 2021 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Emd Serono Inc | TEPMETKO | tepotinib hydrochloride | TABLET;ORAL | 214096-001 | Feb 3, 2021 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Emd Serono Inc | TEPMETKO | tepotinib hydrochloride | TABLET;ORAL | 214096-001 | Feb 3, 2021 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for TEPMETKO
When does loss-of-exclusivity occur for TEPMETKO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 6543
Patent: DERIVADOS DE PIRIDAZINONA
Estimated Expiration: ⤷ Get Started Free
Patent: 7505
Patent: DERIVADOS DE PIRIMIDINIL-PIRIDAZINONA
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 08274534
Patent: Pyrimidinyl pyridazinone derivates
Estimated Expiration: ⤷ Get Started Free
Patent: 08274670
Patent: Pyridazinone derivates
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0813707
Patent: DERIVADOS DE PIRIMIDINIL-PIRIDAZINONA
Estimated Expiration: ⤷ Get Started Free
Patent: 0814616
Patent: DERIVADOS DE PIRIDAZINONA
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 92867
Patent: DÉRIVÉS DE PYRIDAZINONE (Pyrimidinyl-pyridazinone derivatives.)
Estimated Expiration: ⤷ Get Started Free
Patent: 93600
Patent: DERIVES DE PYRIDAZINONE (PYRIDAZINONE DERIVATES)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 08001392
Patent: Compuestos derivados de piridazinona, con actividad moduladora de quinasas; procedimiento de preparacion de dichos compuestos; composicion farmaceutica que comprende a dichos compuestos; kit farmaceutico; y uso para tratar un tumor solido.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1687857
Patent: Pyridazinone derivates
Estimated Expiration: ⤷ Get Started Free
Patent: 1743241
Patent: Pyridazinone derivates
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 70360
Patent: DERIVADOS DE PIRIMIDINIL - PIRIDAZINONA
Estimated Expiration: ⤷ Get Started Free
Patent: 70257
Patent: DERIVADOS DE PIRIDAZINONA
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0120661
Estimated Expiration: ⤷ Get Started Free
Patent: 0150031
Estimated Expiration: ⤷ Get Started Free
Patent: 0170100
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 13137
Estimated Expiration: ⤷ Get Started Free
Patent: 15925
Estimated Expiration: ⤷ Get Started Free
Patent: 18498
Estimated Expiration: ⤷ Get Started Free
Patent: 22017
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 64843
Estimated Expiration: ⤷ Get Started Free
Patent: 64844
Estimated Expiration: ⤷ Get Started Free
Patent: 54660
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 109953
Patent: DERIVADOS DE PIRIDAZINONA
Estimated Expiration: ⤷ Get Started Free
Patent: 109957
Patent: DERIVADOS DE PIRIMIDINIL-PIRIDAZINONA.
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 6782
Patent: ПРОИЗВОДНЫЕ ПИРИДАЗИНОНА (PYRIDAZINONE DERIVATES)
Estimated Expiration: ⤷ Get Started Free
Patent: 7281
Patent: ПРОИЗВОДНЫЕ ПИРИМИДИНИЛПИРИДАЗИНОНА (PYRIMIDINYL PYRIDAZINONE DERIVATES)
Estimated Expiration: ⤷ Get Started Free
Patent: 1000093
Patent: ПРОИЗВОДНЫЕ ПИРИДАЗИНОНА
Estimated Expiration: ⤷ Get Started Free
Patent: 1000094
Patent: ПРОИЗВОДНЫЕ ПИРИМИДИНИЛ-ПИРИДАЗИНОНА
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 64843
Patent: DÉRIVÉS DE PYRIDAZINONE (PYRIDAZINONE DERIVATES)
Estimated Expiration: ⤷ Get Started Free
Patent: 64844
Patent: DÉRIVÉS DE PYRIMIDINYLPYRIDAZINONE (PYRIMIDINYL PYRIDAZINONE DERIVATES)
Estimated Expiration: ⤷ Get Started Free
Patent: 54660
Patent: Dérivés de pyridazinone (Pyridazinone derivatives)
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1024
Estimated Expiration: ⤷ Get Started Free
Germany
Patent: 2007032507
Patent: Pyridazinonderivate
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 42891
Patent: PYRIMIDINYL PYRIDAZINONE DERIVATES
Estimated Expiration: ⤷ Get Started Free
Patent: 45265
Patent: PYRIDAZINONE DERIVATES
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 30519
Estimated Expiration: ⤷ Get Started Free
Patent: 200024
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 3091
Patent: נגזרות פירידאזינון (Pyridazinone derivatives)
Estimated Expiration: ⤷ Get Started Free
Patent: 3094
Patent: נגזרות פירימידיניל-פירידאזינון (Pyrimidinyl-pyridazinone derivatives)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 26543
Estimated Expiration: ⤷ Get Started Free
Patent: 26544
Estimated Expiration: ⤷ Get Started Free
Patent: 10532768
Estimated Expiration: ⤷ Get Started Free
Patent: 10532774
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 2022009
Estimated Expiration: ⤷ Get Started Free
Patent: 54660
Estimated Expiration: ⤷ Get Started Free
Luxembourg
Patent: 0264
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 3727
Patent: PYRIDAZINONE DERIVATIVES
Estimated Expiration: ⤷ Get Started Free
Patent: 3477
Patent: PYRIMIDINYL-PYRIDAZINONE DERIVATIVES
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 1176
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 3186
Patent: PYRIMIDINYL-PYRIDAZINONE DERIVATIVES
Estimated Expiration: ⤷ Get Started Free
Patent: 3187
Patent: PYRIDAZINONE DERIVATIVES
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 22015
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 090287
Patent: DERIVADOS DE PIRIDAZINONA
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 64843
Estimated Expiration: ⤷ Get Started Free
Patent: 64844
Estimated Expiration: ⤷ Get Started Free
Patent: 54660
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 64843
Estimated Expiration: ⤷ Get Started Free
Patent: 64844
Estimated Expiration: ⤷ Get Started Free
Patent: 54660
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 3739
Patent: PYRIDAZINONE DERIVATES
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 64843
Estimated Expiration: ⤷ Get Started Free
Patent: 64844
Estimated Expiration: ⤷ Get Started Free
Patent: 54660
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1001023
Patent: PYRIMIDINYL PYRIDAZINONE DERIVATES
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1544624
Estimated Expiration: ⤷ Get Started Free
Patent: 1553418
Estimated Expiration: ⤷ Get Started Free
Patent: 100031771
Patent: PYRIMIDINYL PYRIDAZINONE DERIVATIVES
Estimated Expiration: ⤷ Get Started Free
Patent: 100050504
Patent: PYRIDAZINONE DERIVATES
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 88883
Estimated Expiration: ⤷ Get Started Free
Patent: 26352
Estimated Expiration: ⤷ Get Started Free
Patent: 14283
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 01768
Estimated Expiration: ⤷ Get Started Free
Patent: 0906409
Patent: Pyridazinone derivatives
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 621
Patent: ПОХІДНІ ПІРИДАЗИНОНУ[ПРОИЗВОДНЫЕ ПИРИДАЗИНОНА (PYRIDAZINONE DERIVATES)
Estimated Expiration: ⤷ Get Started Free
Patent: 833
Patent: ПОХІДНІ ПІРИМІДИНІЛ-ПІРИДАЗИНОНУ[ПРОИЗВОДНЫЕ ПИРИМИДИНИЛ-ПИРИДАЗИНОНА (PYRIMIDINYL PYRIDAZINONE DERIVATES)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TEPMETKO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Norway | 2022015 | ⤷ Get Started Free | |
| European Patent Office | 2754660 | Dérivés de pyridazinone (Pyridazinone derivatives) | ⤷ Get Started Free |
| Eurasian Patent Organization | 017281 | ПРОИЗВОДНЫЕ ПИРИМИДИНИЛПИРИДАЗИНОНА (PYRIMIDINYL PYRIDAZINONE DERIVATES) | ⤷ Get Started Free |
| Taiwan | 200906409 | Pyridazinone derivatives | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TEPMETKO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2164843 | C02164843/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: TEPOTINIB; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 68113 22.06.2021 |
| 2164843 | 122022000047 | Germany | ⤷ Get Started Free | PRODUCT NAME: TEPOTINIB SOWIE DIE PHARMAZEUTISCH VERWENDBAREN SOLVATE, SALZE UND TAUTOMERE DAVON, EINSCHL. DEREN MISCHUNGEN IN ALLEN VERHAELTNISSEN; REGISTRATION NO/DATE: EU/1/21/1596 20220216 |
| 2164843 | 2022C/519 | Belgium | ⤷ Get Started Free | PRODUCT NAME: TEPOTINIB EN FARMACEUTISCH AANVAARDARBE SOLVATEN, ZOUTEN, TAUTOMEREN EN STEREOISOMEREN DAARVAN; AUTHORISATION NUMBER AND DATE: EU/1/21/1596 20220217 |
| 2164843 | PA2022009,C2164843 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: TEPOTINIBAS IR FARMACINIU POZIURIU TINKAMI NAUDOTI JO SOLVATAI, DRUSKOS, TAUTOMERAI IR STEREOIZOMERAI ; REGISTRATION NO/DATE: EU/1/21/1596 20220216 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for TEPMETKO
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
