Actelion Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ACTELION, and when can generic versions of ACTELION drugs launch?
ACTELION has eight approved drugs.
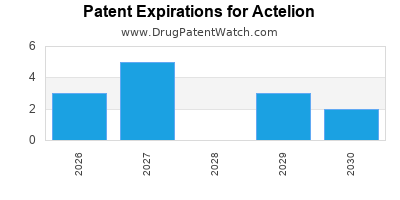
There are fifteen US patents protecting ACTELION drugs.
There are two hundred and forty-seven patent family members on ACTELION drugs in thirty-nine countries and thirty-four supplementary protection certificates in seventeen countries.
Summary for Actelion
| International Patents: | 247 |
| US Patents: | 15 |
| Tradenames: | 6 |
| Ingredients: | 6 |
| NDAs: | 8 |
| PTAB Cases with Actelion as petitioner: | See PTAB cases with Actelion as petitioner |
Drugs and US Patents for Actelion
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actelion | VELETRI | epoprostenol sodium | INJECTABLE;INJECTION | 022260-002 | Jun 28, 2012 | AP2 | RX | Yes | No | 8,598,227 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Actelion | VELETRI | epoprostenol sodium | INJECTABLE;INJECTION | 022260-001 | Jun 27, 2008 | AP2 | RX | Yes | Yes | 8,318,802 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Actelion | UPTRAVI | selexipag | TABLET;ORAL | 207947-005 | Dec 21, 2015 | RX | Yes | No | 10,821,108 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Actelion | TRACLEER | bosentan | TABLET, FOR SUSPENSION;ORAL | 209279-001 | Sep 5, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Actelion | UPTRAVI | selexipag | TABLET;ORAL | 207947-005 | Dec 21, 2015 | RX | Yes | No | 9,284,280 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Actelion | UPTRAVI | selexipag | TABLET;ORAL | 207947-002 | Dec 21, 2015 | AB | RX | Yes | Yes | 9,284,280 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Actelion | UPTRAVI | selexipag | TABLET;ORAL | 207947-003 | Dec 21, 2015 | AB | RX | Yes | No | 9,284,280 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Actelion
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Actelion | TRACLEER | bosentan | TABLET;ORAL | 021290-001 | Nov 20, 2001 | 5,292,740 | ⤷ Try a Trial |
| Actelion | TRACLEER | bosentan | TABLET;ORAL | 021290-002 | Nov 20, 2001 | 5,292,740 | ⤷ Try a Trial |
| Actelion | ZAVESCA | miglustat | CAPSULE;ORAL | 021348-001 | Jul 31, 2003 | 5,472,969 | ⤷ Try a Trial |
| Actelion | ZAVESCA | miglustat | CAPSULE;ORAL | 021348-001 | Jul 31, 2003 | 5,525,616 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for ACTELION drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 10 mg | ➤ Subscribe | 2017-10-18 |
| ➤ Subscribe | for Oral Suspension | 32 mg | ➤ Subscribe | 2019-02-08 |
International Patents for Actelion Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Poland | 2447254 | ⤷ Try a Trial |
| Brazil | 112018009534 | ⤷ Try a Trial |
| Croatia | P20200539 | ⤷ Try a Trial |
| Canada | 3005169 | ⤷ Try a Trial |
| Lithuania | 3300729 | ⤷ Try a Trial |
| Japan | 5956025 | ⤷ Try a Trial |
| Russian Federation | 2010129460 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Actelion Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1345920 | C300672 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: MACITENTAN, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT; REGISTRATION NO/DATE: EU/1/13/893/001-003 20131220 |
| 1345920 | 1490025-2 | Sweden | ⤷ Try a Trial | PRODUCT NAME: MACITENTAN; REG. NO/DATE: EU/1/13/893 20131220 |
| 1400518 | CA 2016 00048 | Denmark | ⤷ Try a Trial | PRODUCT NAME: SELEXIPAG ELLER ET SALT DERAF; REG. NO/DATE: EU/1/15/1083 20160519 |
| 2447254 | 2018015 | Norway | ⤷ Try a Trial | PRODUCT NAME: 2-4-(N-(5,6-DIFENYLPYRAZIN-2- YL)-N- ISOPROPYLAMINO)BUTYLOKSY-N-; REG. NO/DATE: EU/1/15/1083 20160530 |
| 1400518 | 44/2016 | Austria | ⤷ Try a Trial | PRODUCT NAME: SELEXIPAG ODER EIN SALZ DAVON; REGISTRATION NO/DATE: EU/1/15/1083 (MITTEILUNG) 20160519 |
| 1400518 | 132016000106856 | Italy | ⤷ Try a Trial | PRODUCT NAME: SELEXIPAG O UN SUO SALE(UPTRAVI); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/15/1083, 20160519 |
| 1400518 | 532 | Finland | ⤷ Try a Trial | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.