OLYSIO Drug Patent Profile
✉ Email this page to a colleague
When do Olysio patents expire, and when can generic versions of Olysio launch?
Olysio is a drug marketed by Janssen Prods and is included in one NDA. There are nine patents protecting this drug.
This drug has one hundred and forty patent family members in forty-three countries.
The generic ingredient in OLYSIO is simeprevir sodium. There is one drug master file entry for this compound. Additional details are available on the simeprevir sodium profile page.
DrugPatentWatch® Generic Entry Outlook for Olysio
Olysio was eligible for patent challenges on November 22, 2017.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be September 5, 2029. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for OLYSIO?
- What are the global sales for OLYSIO?
- What is Average Wholesale Price for OLYSIO?
Summary for OLYSIO
| International Patents: | 140 |
| US Patents: | 9 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 7 |
| Patent Applications: | 1,537 |
| Drug Prices: | Drug price information for OLYSIO |
| DailyMed Link: | OLYSIO at DailyMed |
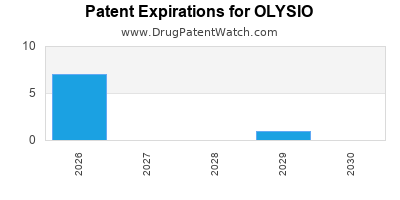

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for OLYSIO
Generic Entry Date for OLYSIO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for OLYSIO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Stanford University | Phase 4 |
| Yale University | Phase 4 |
| Alexion Pharmaceuticals | Phase 1 |
US Patents and Regulatory Information for OLYSIO
OLYSIO is protected by nine US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of OLYSIO is ⤷ Get Started Free.
This potential generic entry date is based on patent 8,148,399.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen Prods | OLYSIO | simeprevir sodium | CAPSULE;ORAL | 205123-001 | Nov 22, 2013 | DISCN | Yes | No | 8,349,869 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Janssen Prods | OLYSIO | simeprevir sodium | CAPSULE;ORAL | 205123-001 | Nov 22, 2013 | DISCN | Yes | No | 9,353,103 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Janssen Prods | OLYSIO | simeprevir sodium | CAPSULE;ORAL | 205123-001 | Nov 22, 2013 | DISCN | Yes | No | 9,040,562 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Janssen Prods | OLYSIO | simeprevir sodium | CAPSULE;ORAL | 205123-001 | Nov 22, 2013 | DISCN | Yes | No | 9,623,022 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Janssen Prods | OLYSIO | simeprevir sodium | CAPSULE;ORAL | 205123-001 | Nov 22, 2013 | DISCN | Yes | No | 8,754,106 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for OLYSIO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Janssen Prods | OLYSIO | simeprevir sodium | CAPSULE;ORAL | 205123-001 | Nov 22, 2013 | 7,671,032 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for OLYSIO
When does loss-of-exclusivity occur for OLYSIO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 06
Patent: Macrocydic inhibitors of hepatitis C virus.
Estimated Expiration: ⤷ Get Started Free
Argentina
Patent: 5359
Patent: INHIBIDORES MACROCICLICOS DEL VIRUS DE LA HEPATITIS C
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0614654
Patent: inibidores macrocìclicos de vìrus de hepatite c
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 16580
Patent: INHIBITEURS MACROCYCLIQUES DU VIRUS DE L'HEPATITE C (MACROCYCLIC INHIBITORS OF HEPATITIS C VIRUS)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2627639
Patent: Macrocydic inhibitors of hepatitis c virus.
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 83
Patent: INHIBIDORES MACROCICLICOS DEL VIRUS DE LA HEPATITIS C
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0151326
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 12006
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 12999
Estimated Expiration: ⤷ Get Started Free
El Salvador
Patent: 08002642
Patent: INHIBIDORES MACROCICLICOS DEL VIRUS DE HEPATITIS C REF. PIT 111 SLV
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 5131
Patent: МАКРОЦИКЛИЧЕСКИЕ ИНГИБИТОРЫ ВИРУСА ГЕПАТИТА С (MACROCYCLIC INHIBITORS OF HEPATITIS C VIRUS)
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 22516
Patent: Composés intermédiaires pour la préparation d'inhibiteurs macrocycliques du virus de l'hépatite C (Intermediates for the preparation of Macrocyclic inhibitors of hepatitis c virus)
Estimated Expiration: ⤷ Get Started Free
Patent: 37339
Patent: Inhibiteurs macrocycliques du virus de l'hépatite C (Macrocylic inhibitors of hepatitis c virus)
Estimated Expiration: ⤷ Get Started Free
Honduras
Patent: 08000134
Patent: INHIBIDORES MACROCICLICOS DEL VIRUS HEPATITIS C
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 83872
Patent: 丙型肝炎病毒的大環抑制劑 (MACROCYLIC INHIBITORS OF HEPATITIS C VIRUS)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 27156
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 8227
Patent: תרכובות מקרוציקליות, שילובים ותכשירים רוקחיים המכילים אותן, שימושים בהן כמעכבי וירוס הפטיטיס c ותהליכים להכנתן (Macrocyclic compounds, combinations and pharmaceutical compositions comprising them, uses thereof as inhibitors of hepatitis c virus and processes for their preparation)
Estimated Expiration: ⤷ Get Started Free
Luxembourg
Patent: 568
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 231
Patent: Makrociklički inhibitori virusa hepatitisa C (MACROCYCLIC INHIBITORS OF HEPATITIS C VIRUS)
Estimated Expiration: ⤷ Get Started Free
Patent: 415
Patent: Intermedijari za pripremu makrocikličkih inhibitora virusa hepatitisa C (Intermediates for the preparation of Macrocyclic inhibitors of hepatitis c virus)
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 12999
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 743
Patent: MAKROCIKLIČKI INHIBITORI VIRUSA HEPATITISA C (MACROCYCLIC INHIBITORS OF HEPATITIS C VIRUS)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 22516
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 080042084
Patent: MACROCYLIC INHIBITORS OF HEPATITIS C VIRUS
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 245
Patent: МАКРОЦИКЛИЧЕСКИЕ ИНГИБИТОРЫ ВИРУСА ГЕПАТИТА С;МАКРОЦИКЛІЧНІ ІНГІБІТОРИ ВІРУСУ ГЕПАТИТУ С (MACROCYCLIC INHIBITORS OF HEPATITIS C VIRUS)
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 703
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering OLYSIO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Portugal | 1713823 | ⤷ Get Started Free | |
| Spain | 2342944 | ⤷ Get Started Free | |
| Eurasian Patent Organization | 200601400 | ИНГИБИТОРЫ NS-3 СЕРИНОВОЙ ПРОТЕАЗЫ HCV | ⤷ Get Started Free |
| Taiwan | I358411 | ⤷ Get Started Free | |
| World Intellectual Property Organization (WIPO) | 2005073216 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for OLYSIO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1713823 | C300703 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: SIMEPREVIR, OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN, WAARONDER SIMEPREVIRNATRIUM; REGISTRATION NO/DATE: EU/1/14/924/001-002 20140514 |
| 1912999 | 122014000098 | Germany | ⤷ Get Started Free | PRODUCT NAME: SIMEPREVIR ODER EIN SALZ DAVON; REGISTRATION NO/DATE: EU/1/14/924 20140514 |
| 1912999 | 1490062-5 | Sweden | ⤷ Get Started Free | PRODUCT NAME: SIMEPREVIR, OR A SALT THEREOF, INCLUDING SIMEPREVIR SODIUM; REG. NO/DATE: EU/1/14/924 20140516 |
| 1912999 | CA 2014 00053 | Denmark | ⤷ Get Started Free | PRODUCT NAME: SIMEPREVIR ELLER ET SALT DERAF, HERUNDER SIMEPREVIRNATRIUM; REG. NO/DATE: EU/1/14/924 20140514 |
| 1912999 | PA2014036,C1912999 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: SIMEPREVIRAS; REGISTRATION NO/DATE: EU/1/14/924 20140514 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for OLYSIO (Simeprevir)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
