BYDUREON Drug Patent Profile
✉ Email this page to a colleague
When do Bydureon patents expire, and what generic alternatives are available?
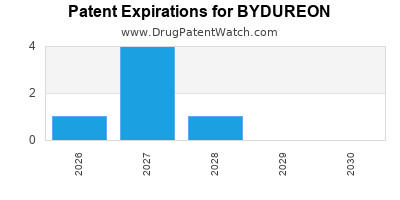
Bydureon is a drug marketed by Astrazeneca Ab and is included in two NDAs. There are eleven patents protecting this drug.
This drug has three hundred and forty-six patent family members in forty-eight countries.
The generic ingredient in BYDUREON is exenatide synthetic. There are seven drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the exenatide synthetic profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Bydureon
A generic version of BYDUREON was approved as exenatide synthetic by AMNEAL on November 19th, 2024.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for BYDUREON?
- What are the global sales for BYDUREON?
- What is Average Wholesale Price for BYDUREON?
Summary for BYDUREON
| International Patents: | 346 |
| US Patents: | 10 |
| Applicants: | 1 |
| NDAs: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 29 |
| Clinical Trials: | 40 |
| Patent Applications: | 220 |
| Drug Prices: | Drug price information for BYDUREON |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BYDUREON |
| What excipients (inactive ingredients) are in BYDUREON? | BYDUREON excipients list |
| DailyMed Link: | BYDUREON at DailyMed |

Recent Clinical Trials for BYDUREON
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Christopher D. Verrico | PHASE1 |
| The University of Texas Health Science Center, Houston | Phase 2 |
| University of Washington | Phase 3 |
US Patents and Regulatory Information for BYDUREON
BYDUREON is protected by ten US patents and two FDA Regulatory Exclusivities.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | DISCN | Yes | No | 8,329,648*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | DISCN | Yes | No | 8,501,698*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | DISCN | Yes | No | 7,612,176*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Astrazeneca Ab | BYDUREON PEN | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-002 | Feb 28, 2014 | DISCN | Yes | No | 9,884,092*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Astrazeneca Ab | BYDUREON | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-001 | Jan 27, 2012 | DISCN | Yes | No | 7,456,254*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BYDUREON
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | BYDUREON | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-001 | Jan 27, 2012 | 6,936,590 | ⤷ Get Started Free |
| Astrazeneca Ab | BYDUREON | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-001 | Jan 27, 2012 | 7,741,269 | ⤷ Get Started Free |
| Astrazeneca Ab | BYDUREON | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-001 | Jan 27, 2012 | 5,424,286 | ⤷ Get Started Free |
| Astrazeneca Ab | BYDUREON | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-001 | Jan 27, 2012 | 6,667,061 | ⤷ Get Started Free |
| Astrazeneca Ab | BYDUREON | exenatide synthetic | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 022200-001 | Jan 27, 2012 | 6,495,164 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for BYDUREON
When does loss-of-exclusivity occur for BYDUREON?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 1730
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 07265246
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0713544
Estimated Expiration: ⤷ Get Started Free
Patent: 2017015106
Estimated Expiration: ⤷ Get Started Free
Patent: 2017021516
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 53344
Estimated Expiration: ⤷ Get Started Free
Patent: 24318
Estimated Expiration: ⤷ Get Started Free
Patent: 85797
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 07001915
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1479287
Estimated Expiration: ⤷ Get Started Free
Patent: 3145773
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 60299
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0141007
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 15738
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 69374
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 8229
Estimated Expiration: ⤷ Get Started Free
Patent: 0428
Estimated Expiration: ⤷ Get Started Free
Patent: 8259
Estimated Expiration: ⤷ Get Started Free
Patent: 5999
Estimated Expiration: ⤷ Get Started Free
Patent: 0900066
Estimated Expiration: ⤷ Get Started Free
Patent: 1171333
Estimated Expiration: ⤷ Get Started Free
Patent: 1490902
Estimated Expiration: ⤷ Get Started Free
Patent: 1791254
Estimated Expiration: ⤷ Get Started Free
Patent: 2091391
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 69374
Estimated Expiration: ⤷ Get Started Free
Patent: 57918
Estimated Expiration: ⤷ Get Started Free
Patent: 45466
Estimated Expiration: ⤷ Get Started Free
Patent: 63807
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 27359
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 5882
Estimated Expiration: ⤷ Get Started Free
Patent: 4180
Estimated Expiration: ⤷ Get Started Free
Patent: 4181
Estimated Expiration: ⤷ Get Started Free
Patent: 4182
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 13889
Estimated Expiration: ⤷ Get Started Free
Patent: 66651
Estimated Expiration: ⤷ Get Started Free
Patent: 37187
Estimated Expiration: ⤷ Get Started Free
Patent: 09545525
Estimated Expiration: ⤷ Get Started Free
Patent: 13209394
Estimated Expiration: ⤷ Get Started Free
Patent: 15071636
Estimated Expiration: ⤷ Get Started Free
Patent: 16172758
Estimated Expiration: ⤷ Get Started Free
Patent: 17222681
Estimated Expiration: ⤷ Get Started Free
Patent: 19059779
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 8566
Estimated Expiration: ⤷ Get Started Free
Patent: 3930
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 9143
Estimated Expiration: ⤷ Get Started Free
Patent: 7155
Estimated Expiration: ⤷ Get Started Free
Patent: 08015377
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 4346
Estimated Expiration: ⤷ Get Started Free
Patent: 9190
Estimated Expiration: ⤷ Get Started Free
Patent: 9195
Estimated Expiration: ⤷ Get Started Free
Patent: 9202
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 6828
Estimated Expiration: ⤷ Get Started Free
Patent: 7770
Estimated Expiration: ⤷ Get Started Free
Patent: 085169
Estimated Expiration: ⤷ Get Started Free
Patent: 221233
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 080349
Estimated Expiration: ⤷ Get Started Free
Patent: 120776
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 012500168
Patent: CRYSTALLINE SOLVATES AND COMPLEXES OF (IS)-1,5-ANHYDRO-L-C-(3-((PHENYL)METHYL)PHENYL)-D-GLUCITOL DERIVATIVES WITH AMINO ACIDS AS SGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 69374
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 69374
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 638
Patent: KRISTALNI SOLVATI DERIVATA (1S)-1,5-ANHIDRO-1-C-(3-((FENIL) METIL) FENIL)-D-GLUCITOLA SA ALKOHOLIMA KAO INHIBITORI SGLT2 ZA TRETMAN DIJABETESA (CRYSTALLINE SOLVATES OF (1S)-1,5-ANHYDRO-1-C-(3-((PHENYL) METHYL) PHENYL)-D-GLUCITOL DERIVATIVES WITH ALCOHOLS AS SGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 2741
Patent: CRYSTALLINE SOLVATES AND COMPLEXES OF (1S) -1, 5-ANHYDRO-1-C- (3- ( (PHENYL) METHYL) PHENYL) -D-GLUCITOL DERIVATIVES WITH AMINO ACIDS AS SGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES
Estimated Expiration: ⤷ Get Started Free
Patent: 201402181S
Patent: CRYSTALLINE SOLVATES AND COMPLEXES OF (1S) -1, 5-ANHYDRO-1-C- (3- ( (PHENYL) METHYL) PHENYL) -D-GLUCITOL DERIVATIVES WITH AMINO ACIDS AS SGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 69374
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0810475
Patent: CRYSTALLINE SOLVATES AND COMPLEXES OF (IS)-1,5-ANHYDRO-L-C-(3-((PHENYL)METHYL)PHENYL)-D-GLUCITOL DERIVATIVES WITH AMINO ACIDS AS SGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1493102
Estimated Expiration: ⤷ Get Started Free
Patent: 090023643
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 21665
Estimated Expiration: ⤷ Get Started Free
Patent: 59862
Estimated Expiration: ⤷ Get Started Free
Patent: 69130
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 21245
Estimated Expiration: ⤷ Get Started Free
Patent: 66876
Estimated Expiration: ⤷ Get Started Free
Patent: 19528
Estimated Expiration: ⤷ Get Started Free
Patent: 0811127
Patent: Crystal structures of SGLT2 inhibitors and processes for preparing same
Estimated Expiration: ⤷ Get Started Free
Patent: 1406743
Patent: Crystal structures of SGLT2 inhibitors and processes for preparing same
Estimated Expiration: ⤷ Get Started Free
Patent: 1509927
Patent: Crystal structures of SGLT2 inhibitors and processes for preparing same
Estimated Expiration: ⤷ Get Started Free
Patent: 1546054
Patent: Crystal structures of SGLT2 inhibitors and processes for preparing same
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 765
Patent: КРИСТАЛЛИЧЕСКИЕ СОЛЬВАТЫ И КОМПЛЕКСЫ ПРОИЗВОДНЫХ (IS)-1,5-АНГИДРО-L-C-(3-((ФЕНИЛ)МЕТИЛ)ФЕНИЛ)-D-ГЛЮЦИТОЛА С АМИНОКИСЛОТАМИ КАК ИНГИБИТОРЫ БЕЛКА SGLT2, ПРИГОДНЫЕ В ЛЕЧЕНИИ ДИАБЕТА;КРИСТАЛІЧНІ СОЛЬВАТИ І КОМПЛЕКСИ ПОХІДНИХ (IS)-1,5-АНГІДРО-L-C-(3-((ФЕНІЛ)МЕТИЛ)ФЕНІЛ)-D-ГЛЮЦИТОЛУ З АМІНОКИСЛОТАМИ ЯК ІНГІБІТОРИ БІЛКА SGLT2, ПРИДАТНІ У ЛІКУВАННІ ДІАБЕТУ (CRYSTALLINE SOLVATES AND COMPLEXES OF (IS) -1, 5-ANHYDRO-L-C- (3- ((PHENYL) METHYL) PHENYL) -D-GLUCITOL DERIVATIVES WITH AMINO ACIDS AS SGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering BYDUREON around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Mexico | 2009010155 | FORMULACIONES FARMACEUTICAS QUE CONTIENEN HIDRATO DE PROPILENGLICOL DE DAPAGLIFLOZINA. (PHARMACEUTICAL FORMULATIONS CONTAINING DAPAGLIFLOZIN PROPYLENE GLYCOL HYDRATE.) | ⤷ Get Started Free |
| South Korea | 101021752 | ⤷ Get Started Free | |
| Germany | 102008025002 | Abmischvorrichtung für eine Zweikammerampulle | ⤷ Get Started Free |
| Norway | 2010010 | ⤷ Get Started Free | |
| Peru | 20040760 | C-ARIL-GLUCOSIDOS COMO INHIBIDORES DE SGLT2 | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BYDUREON
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0996459 | 464 | Finland | ⤷ Get Started Free | |
| 1734971 | CA 2012 00015 | Denmark | ⤷ Get Started Free | PRODUCT NAME: EXENATIDE; REG. NO/DATE: EU/1/11/696/001-002 20110623 |
| 1506211 | SPC/GB13/021 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: DAPAGLIFLOZIN AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTERED: UK EU/1/12/795/001 20121114; UK EU/1/12/795/002 20121114; UK EU/1/12/795/003 20121114; UK EU/1/12/795/004 20121114; UK EU/1/12/795/005 20121114; UK EU/1/12/795/006 20121114; UK EU/1/12/795/007 20121114; UK EU/1/12/795/008 20121114; UK EU/1/12/795/009 20121114; UK EU/1/12/795/010 20121114 |
| 2139494 | 301054 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: SAXAGLIPTIN AND DAPAGLIFLOZIN; REGISTRATION NO/DATE: EU/1/16/1108 20160719 |
| 1140145 | SZ 30/2007 | Austria | ⤷ Get Started Free | PRODUCT NAME: EXENATIDE |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
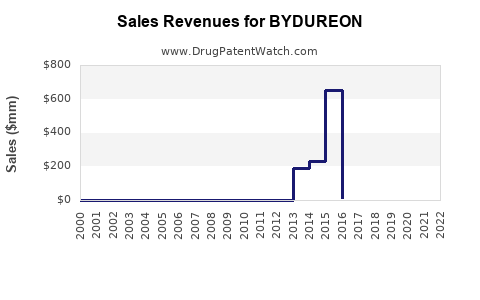
Market Dynamics and Financial Trajectory for BYDUREON
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
