TWIRLA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Twirla, and what generic alternatives are available?
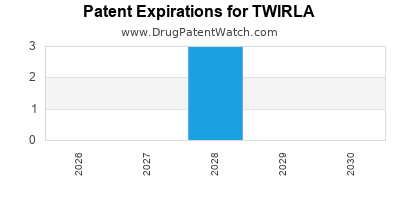
Twirla is a drug marketed by Agile and is included in one NDA. There are three patents protecting this drug.
This drug has nineteen patent family members in twelve countries.
The generic ingredient in TWIRLA is ethinyl estradiol; levonorgestrel. There are twenty-six drug master file entries for this compound. Twenty-two suppliers are listed for this compound. Additional details are available on the ethinyl estradiol; levonorgestrel profile page.
DrugPatentWatch® Generic Entry Outlook for Twirla
There are two tentative approvals for the generic drug (ethinyl estradiol; levonorgestrel), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TWIRLA?
- What are the global sales for TWIRLA?
- What is Average Wholesale Price for TWIRLA?
Summary for TWIRLA
| International Patents: | 19 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 5 |
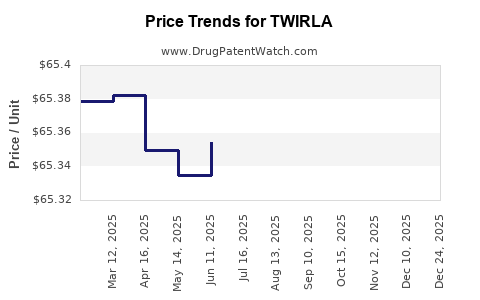
| Drug Prices: | Drug price information for TWIRLA |
| What excipients (inactive ingredients) are in TWIRLA? | TWIRLA excipients list |
| DailyMed Link: | TWIRLA at DailyMed |
Pharmacology for TWIRLA
| Drug Class | Estrogen Progestin Progestin-containing Intrauterine System |
| Mechanism of Action | Estrogen Receptor Agonists |
| Physiological Effect | Inhibit Ovum Fertilization |
US Patents and Regulatory Information for TWIRLA
TWIRLA is protected by three US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agile | TWIRLA | ethinyl estradiol; levonorgestrel | SYSTEM;TRANSDERMAL | 204017-001 | Feb 14, 2020 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Agile | TWIRLA | ethinyl estradiol; levonorgestrel | SYSTEM;TRANSDERMAL | 204017-001 | Feb 14, 2020 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Agile | TWIRLA | ethinyl estradiol; levonorgestrel | SYSTEM;TRANSDERMAL | 204017-001 | Feb 14, 2020 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TWIRLA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Agile | TWIRLA | ethinyl estradiol; levonorgestrel | SYSTEM;TRANSDERMAL | 204017-001 | Feb 14, 2020 | ⤷ Get Started Free | ⤷ Get Started Free |
| Agile | TWIRLA | ethinyl estradiol; levonorgestrel | SYSTEM;TRANSDERMAL | 204017-001 | Feb 14, 2020 | ⤷ Get Started Free | ⤷ Get Started Free |
| Agile | TWIRLA | ethinyl estradiol; levonorgestrel | SYSTEM;TRANSDERMAL | 204017-001 | Feb 14, 2020 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TWIRLA
When does loss-of-exclusivity occur for TWIRLA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 08275101
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0814697
Patent: dispositivo de liberação de fármacos
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 92884
Patent: DISPOSITIF D'ADMINISTRATION DERMIQUE AVEC UN JOINTIN SITU (Dermal delivery device with in situ seal)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1801321
Patent: Dermal delivery device with in situ seal
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 0208
Patent: УСТРОЙСТВО ДОСТАВКИ В КОЖУ С ИЗОЛИРУЮЩИМ СЛОЕМ IN SITU (DERMAL DELIVERY DEVICE WITH IN SITU SEAL)
Estimated Expiration: ⤷ Get Started Free
Patent: 1070123
Patent: УСТРОЙСТВО ДОСТАВКИ В КОЖУ С ИЗОЛИРУЮЩИМ СЛОЕМ IN SITU
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 67001
Patent: DISPOSITIF D'ADMINISTRATION THERMIQUE AVEC SOUDURE ULTRASONORE (DERMAL DELIVERY DEVICE WITH ULTRASONIC WELD)
Estimated Expiration: ⤷ Get Started Free
Patent: 67002
Patent: DISPOSITIF D'ADMINISTRATION DERMIQUE AVEC UN JOINT IN SITU (DERMAL DELIVERY DEVICE WITH IN SITU SEAL)
Estimated Expiration: ⤷ Get Started Free
Georgia, Republic of
Patent: 0125717
Patent: DEVICE FOR MEDICINE DIRECT DERMAL DELIVERY
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 42798
Patent: 具有原位密封的皮膚遞送裝置 (DERMAL DELIVERY DEVICE WITH IN SITU SEAL)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 03235
Estimated Expiration: ⤷ Get Started Free
Patent: 72725
Estimated Expiration: ⤷ Get Started Free
Patent: 10533199
Estimated Expiration: ⤷ Get Started Free
Patent: 14159468
Patent: DERMAL DELIVERY DEVICE WITH IN SITU SEAL
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 2565
Patent: DERMAL DELIVERY DEVICE WITH IN SITU SEAL
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 81652
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TWIRLA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Mexico | PA02005224 | SISTEMA Y PROCEDIMIENTO MEJORADO PARA LA ADMINISTRACION TRANSDERMICA DE UN ANTICONCEPTIVO. (IMPROVED TRANSDERMAL CONTRACEPTIVE DELIVERY SYSTEM AND PROCESS.) | ⤷ Get Started Free |
| World Intellectual Property Organization (WIPO) | 0137770 | ⤷ Get Started Free | |
| Norway | 331218 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TWIRLA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0136011 | 2000C/027 | Belgium | ⤷ Get Started Free | PRODUCT NAME: ETHINYLESTRADIOLUM / NORETHISTERONI ACETAS; NAT. REGISTRATION NO/DATE: 19 IS 106 F3 20000911; FIRST REGISTRATION: NL RVG 23909 19991124 |
| 0771217 | 07C0001 | France | ⤷ Get Started Free | PRODUCT NAME: ETHINYLESTRADIOL BETADEX CLATHRATE; NAT. REGISTRATION NO/DATE: NL 32343 20060710; FIRST REGISTRATION: NL - RVG 31781 20050804 |
| 1380301 | CA 2009 00017 | Denmark | ⤷ Get Started Free | PRODUCT NAME: ETHINYLESTRADIOL (SOM BETADEXCLATHRAT) OG DROSPIRENON; NAT. REG. NO/DATE: 42417 (DK) 20080619; FIRST REG. NO/DATE: NL 33842 20070629 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for TWIRLA: An In-depth Analysis
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
