VELPHORO Drug Patent Profile
✉ Email this page to a colleague
When do Velphoro patents expire, and what generic alternatives are available?
Velphoro is a drug marketed by Vifor Fresenius and is included in one NDA. There are eleven patents protecting this drug.
This drug has one hundred and thirty-five patent family members in thirty-six countries.
The generic ingredient in VELPHORO is ferric oxyhydroxide. There are twenty drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the ferric oxyhydroxide profile page.
DrugPatentWatch® Generic Entry Outlook for Velphoro
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be July 23, 2030. This may change due to patent challenges or generic licensing.
There have been seven patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for VELPHORO?
- What are the global sales for VELPHORO?
- What is Average Wholesale Price for VELPHORO?
Summary for VELPHORO
| International Patents: | 135 |
| US Patents: | 11 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 4 |
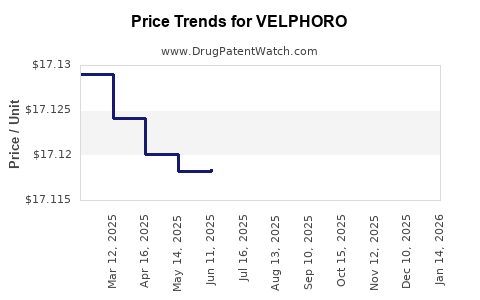
| Drug Prices: | Drug price information for VELPHORO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for VELPHORO |
| What excipients (inactive ingredients) are in VELPHORO? | VELPHORO excipients list |
| DailyMed Link: | VELPHORO at DailyMed |
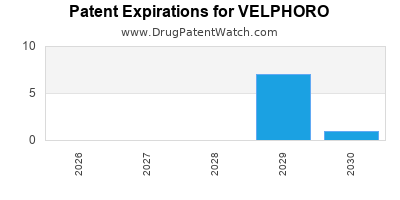
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for VELPHORO
Generic Entry Date for VELPHORO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET, CHEWABLE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for VELPHORO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Colorado, Denver | Phase 4 |
| Vifor Fresenius Medical Care Renal Pharma | Phase 2 |
| Prim. Priv. Doz. Dr. Daniel Cejka | Phase 2 |
Pharmacology for VELPHORO
| Drug Class | Phosphate Binder |
| Mechanism of Action | Phosphate Chelating Activity |
US Patents and Regulatory Information for VELPHORO
VELPHORO is protected by eleven US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of VELPHORO is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vifor Fresenius | VELPHORO | ferric oxyhydroxide | TABLET, CHEWABLE;ORAL | 205109-001 | Nov 27, 2013 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Vifor Fresenius | VELPHORO | ferric oxyhydroxide | TABLET, CHEWABLE;ORAL | 205109-001 | Nov 27, 2013 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Vifor Fresenius | VELPHORO | ferric oxyhydroxide | TABLET, CHEWABLE;ORAL | 205109-001 | Nov 27, 2013 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Vifor Fresenius | VELPHORO | ferric oxyhydroxide | TABLET, CHEWABLE;ORAL | 205109-001 | Nov 27, 2013 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Vifor Fresenius | VELPHORO | ferric oxyhydroxide | TABLET, CHEWABLE;ORAL | 205109-001 | Nov 27, 2013 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for VELPHORO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Vifor Fresenius | VELPHORO | ferric oxyhydroxide | TABLET, CHEWABLE;ORAL | 205109-001 | Nov 27, 2013 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for VELPHORO
When does loss-of-exclusivity occur for VELPHORO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 9312
Patent: COMPOSICION FARMACEUTICA QUE COMPRENDE BETA OXIHIDROXIDO DE HIERRO EN ALTA CARGA EN FORMA ADECUADA PARA ADMINISTARCION ORAL Y QUE CONTIENE AL MENOS UN HIDRATO DE CARBONO Y/O ACIDO HUMICO Y SU USO EN EL TRATAMIENTO DE PACIENTES CON HIPERFOSFATEMIA Y CON INSUFICIENCIA RENAL CRONICA , Y PROCEDIMENTO PA
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 08322963
Patent: Pharmaceutical compositions
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0820308
Patent: Composições farmacêuticas
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 00444
Patent: COMPOSITIONS PHARMACEUTIQUES RENFERMANT UN OXYHYDROXYDE DE FER A FORTE CHARGE (PHARMACEUTICAL COMPOSITIONS COMPRISING IRON OXY-HYDROXIDE IN HIGH LOADING)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1861146
Patent: Pharmaceutical compositions
Estimated Expiration: ⤷ Get Started Free
Patent: 4688702
Patent: PHARMACEUTICAL COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 6619710
Patent: 药物组合物 (Pharmaceutical compositions)
Estimated Expiration: ⤷ Get Started Free
Patent: 1789820
Patent: 药物组合物 (PHARMACEUTICAL COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 2022796
Patent: 药物组合物 (Pharmaceutical compositions)
Estimated Expiration: ⤷ Get Started Free
Patent: 2022821
Patent: 药物组合物 (Pharmaceutical compositions)
Estimated Expiration: ⤷ Get Started Free
Patent: 2022822
Patent: 药物组合物 (Pharmaceutical compositions)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 80398
Patent: COMPOSICIONES FARMACEUTICAS QUE CONTIENEN OXIHIDROXIDO DE HIERRO EN ALTA CARGA
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0170034
Estimated Expiration: ⤷ Get Started Free
Patent: 0181837
Estimated Expiration: ⤷ Get Started Free
Patent: 0220318
Estimated Expiration: ⤷ Get Started Free
Patent: 0230588
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 18496
Estimated Expiration: ⤷ Get Started Free
Patent: 21230
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 22285
Estimated Expiration: ⤷ Get Started Free
Patent: 43992
Estimated Expiration: ⤷ Get Started Free
Patent: 92069
Estimated Expiration: ⤷ Get Started Free
Patent: 95699
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 22285
Patent: COMPOSITIONS PHARMACEUTIQUES (PHARMACEUTICAL COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 43992
Patent: COMPOSITIONS PHARMACEUTIQUES COMPRENNANT OXY-HYDROXIDE DE FER (PHARMACEUTICAL COMPOSITIONS COMPRISING IRON OXY-HYDROXIDE)
Estimated Expiration: ⤷ Get Started Free
Patent: 92069
Patent: COMPOSITIONS PHARMACEUTIQUES (PHARMACEUTICAL COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 95698
Patent: COMPOSITIONS PHARMACEUTIQUES CONTENANT DE L'OXYHROXYDE DE FER (PHARMACEUTICAL COMPOSITIONS COMPRISING IRON OXY-HYDROXIDE)
Estimated Expiration: ⤷ Get Started Free
Patent: 95699
Patent: COMPOSITIONS PHARMACEUTIQUES CONTENANT DE L'OXYHROXYDE DE FER (PHARMACEUTICAL COMPOSITIONS COMPRISING IRON OXY-HYDROXIDE)
Estimated Expiration: ⤷ Get Started Free
Patent: 95700
Patent: COMPOSITIONS PHARMACEUTIQUES CONTENANT DE L'OXYHYDROXYDE DE FER (PHARMACEUTICAL COMPOSITIONS COMPRISING IRON OXY-HYDROXIDE)
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 95699
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 1000144
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 07298
Patent: 藥物組合物 (PHARMACEUTICAL COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 31293
Estimated Expiration: ⤷ Get Started Free
Patent: 41429
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 5726
Patent: תכשירי רוקחות המיכילים אוקסי-הידרוקסיד של ברזל (Pharmaceutical compositions comprising iron oxy-hydroxide)
Estimated Expiration: ⤷ Get Started Free
Patent: 7741
Patent: תכשירי רוקחות המכילים ברזל אוקסו - הידרוקסיד, שיטות להכנתם והשימושים בהם (Pharmaceutical compositions comprising iron oxy-hydroxide, methods for the preparation thereof and their uses)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 66164
Estimated Expiration: ⤷ Get Started Free
Patent: 61601
Estimated Expiration: ⤷ Get Started Free
Patent: 94260
Estimated Expiration: ⤷ Get Started Free
Patent: 38734
Estimated Expiration: ⤷ Get Started Free
Patent: 64959
Estimated Expiration: ⤷ Get Started Free
Patent: 16055
Estimated Expiration: ⤷ Get Started Free
Patent: 11503148
Estimated Expiration: ⤷ Get Started Free
Patent: 14062120
Patent: PHARMACEUTICAL COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 15166359
Patent: 医薬組成物 (PHARMACEUTICAL COMPOSITION)
Estimated Expiration: ⤷ Get Started Free
Patent: 17114873
Patent: 医薬組成物 (PHARMACEUTICAL COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 19089805
Patent: 医薬組成物 (PHARMACEUTICAL COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 21001192
Patent: 医薬組成物 (PHARMACEUTICAL COMPOSITIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 23011873
Patent: 医薬組成物
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 22285
Estimated Expiration: ⤷ Get Started Free
Patent: 43992
Estimated Expiration: ⤷ Get Started Free
Patent: 92069
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 6383
Patent: PHARMACEUTICAL COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 3729
Patent: PHARMACEUTICAL COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 10005346
Patent: COMPOSICIONES FARMACEUTICAS. (PHARMACEUTICAL COMPOSITIONS.)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 898
Patent: تراكيب صيدلانية
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 5435
Patent: PHARMACEUTICAL COMPOSITIONS COMPRISING IRON OXY-HYDROXIDE
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 015500727
Patent: PHARMACEUTICAL COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 22285
Estimated Expiration: ⤷ Get Started Free
Patent: 43992
Estimated Expiration: ⤷ Get Started Free
Patent: 92069
Estimated Expiration: ⤷ Get Started Free
Patent: 95699
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 22285
Estimated Expiration: ⤷ Get Started Free
Patent: 43992
Estimated Expiration: ⤷ Get Started Free
Patent: 92069
Estimated Expiration: ⤷ Get Started Free
Patent: 95699
Estimated Expiration: ⤷ Get Started Free
Patent: 95700
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 93831
Estimated Expiration: ⤷ Get Started Free
Patent: 48760
Estimated Expiration: ⤷ Get Started Free
Patent: 10124424
Estimated Expiration: ⤷ Get Started Free
Patent: 13128356
Estimated Expiration: ⤷ Get Started Free
Saudi Arabia
Patent: 2340044
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 8789
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 22285
Estimated Expiration: ⤷ Get Started Free
Patent: 43992
Estimated Expiration: ⤷ Get Started Free
Patent: 92069
Estimated Expiration: ⤷ Get Started Free
Patent: 95699
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1004256
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1438071
Estimated Expiration: ⤷ Get Started Free
Patent: 1590115
Estimated Expiration: ⤷ Get Started Free
Patent: 100126266
Patent: PHARMACEUTICAL COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 130030306
Patent: PHARMACEUTICAL COMPOSITIONS
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 12331
Estimated Expiration: ⤷ Get Started Free
Patent: 03158
Estimated Expiration: ⤷ Get Started Free
Patent: 08719
Estimated Expiration: ⤷ Get Started Free
Patent: 50337
Estimated Expiration: ⤷ Get Started Free
Patent: 51457
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 68167
Estimated Expiration: ⤷ Get Started Free
Patent: 92159
Estimated Expiration: ⤷ Get Started Free
Patent: 0938210
Estimated Expiration: ⤷ Get Started Free
Patent: 1509423
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 10000152
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering VELPHORO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Denmark | 3895699 | ⤷ Get Started Free | |
| Hungary | E051111 | ⤷ Get Started Free | |
| Japan | 6764959 | ⤷ Get Started Free | |
| Spain | 2950337 | ⤷ Get Started Free | |
| Tunisia | 2010000152 | PHARMACEUTICAL COMPOSITIONS | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VELPHORO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0868125 | CA 2015 00007 | Denmark | ⤷ Get Started Free | PRODUCT NAME: BLANDING AF POLYNUKLEAERT JERN(III)-OXYHYDROXID, SUCROSE OG STIVELSE; REG. NO/DATE: EU/1/14/943/001-004 20140826 |
| 0868125 | C00868125/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: FERRIOXYHYDROXIDUM/SACCHARUM/AMYLA; REGISTRATION NO/DATE: SWISSMEDIC 62986 22.01.2015 |
| 0868125 | SPC/GB14/087 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: MIXTURE OF POLYNUCLEAR IRON(III)OXYHYDROXIDE, SUCROSE AND STARCHES; REGISTERED: UK EU/1/14/943/001 20140828; UK EU/1/14/943/002 20140828; UK EU/1/14/943/003 20140828; UK EU/1/14/943/004 20140828 |
| 0868125 | 15C0018 | France | ⤷ Get Started Free | PRODUCT NAME: MELANGE D'OXYHYDROXYDE DE FER (III) POLYNUCLEAIRE,DE SACCHAROSE ET D'AMIDONS.; REGISTRATION NO/DATE: EU/1/14/943/001 20140826 |
| 0868125 | 425 | Finland | ⤷ Get Started Free | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for VELPHORO
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
