EVOTAZ Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Evotaz, and when can generic versions of Evotaz launch?
Evotaz is a drug marketed by Bristol and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has three hundred and five patent family members in forty-one countries.
The generic ingredient in EVOTAZ is atazanavir sulfate; cobicistat. There are twenty-five drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the atazanavir sulfate; cobicistat profile page.
DrugPatentWatch® Generic Entry Outlook for Evotaz
Evotaz was eligible for patent challenges on August 27, 2016.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be October 6, 2032. This may change due to patent challenges or generic licensing.
There have been eight patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for EVOTAZ?
- What are the global sales for EVOTAZ?
- What is Average Wholesale Price for EVOTAZ?
Summary for EVOTAZ
| International Patents: | 305 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 5 |
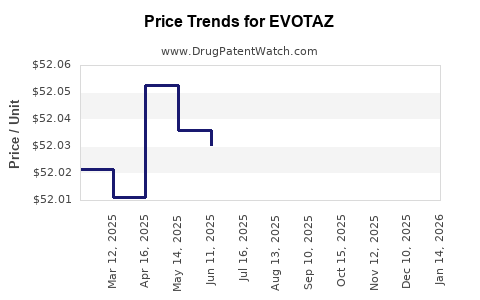
| Drug Prices: | Drug price information for EVOTAZ |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for EVOTAZ |
| What excipients (inactive ingredients) are in EVOTAZ? | EVOTAZ excipients list |
| DailyMed Link: | EVOTAZ at DailyMed |
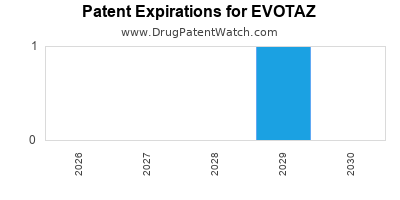
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for EVOTAZ
Generic Entry Date for EVOTAZ*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for EVOTAZ
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Gilead Sciences | Phase 3 |
| Bristol-Myers Squibb | Phase 1 |
| St Stephens Aids Trust | Phase 1 |
Pharmacology for EVOTAZ
Paragraph IV (Patent) Challenges for EVOTAZ
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| EVOTAZ | Tablets | atazanavir sulfate; cobicistat | 300 mg/150 mg | 206353 | 1 | 2017-09-13 |
US Patents and Regulatory Information for EVOTAZ
EVOTAZ is protected by two US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of EVOTAZ is ⤷ Get Started Free.
This potential generic entry date is based on patent 10,039,718.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bristol | EVOTAZ | atazanavir sulfate; cobicistat | TABLET;ORAL | 206353-001 | Jan 29, 2015 | RX | Yes | Yes | 8,148,374 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Bristol | EVOTAZ | atazanavir sulfate; cobicistat | TABLET;ORAL | 206353-001 | Jan 29, 2015 | RX | Yes | Yes | 10,039,718 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for EVOTAZ
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bristol | EVOTAZ | atazanavir sulfate; cobicistat | TABLET;ORAL | 206353-001 | Jan 29, 2015 | 5,849,911*PED | ⤷ Get Started Free |
| Bristol | EVOTAZ | atazanavir sulfate; cobicistat | TABLET;ORAL | 206353-001 | Jan 29, 2015 | 6,087,383*PED | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for EVOTAZ
When does loss-of-exclusivity occur for EVOTAZ?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 89
Patent: The use of solid carrier particles to improve the processability of a pharmaceutical agent
Estimated Expiration: ⤷ Get Started Free
Patent: 50
Patent: Tablets for combination therapy
Estimated Expiration: ⤷ Get Started Free
Argentina
Patent: 5369
Patent: COMPRIMIDOS PARA TERAPIA DE COMBINACION PARA EL TRATAMIENTO DE INFECCIONES VIRALES.
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 09242451
Patent: The use of solid carrier particles to improve the processability of a pharmaceutical agent
Estimated Expiration: ⤷ Get Started Free
Patent: 10210598
Patent: Tablets for combination therapy
Estimated Expiration: ⤷ Get Started Free
Patent: 14221210
Estimated Expiration: ⤷ Get Started Free
Patent: 15200637
Patent: TABLETS FOR COMBINATION THERAPY
Estimated Expiration: ⤷ Get Started Free
Patent: 16250470
Patent: THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT
Estimated Expiration: ⤷ Get Started Free
Patent: 17201473
Patent: Tablets for combination therapy
Estimated Expiration: ⤷ Get Started Free
Patent: 18267573
Patent: Tablets for combination therapy
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0911871
Patent: uso de partículas veículo sólidas para melhorar a processabilidade de um agente farmacêutico
Estimated Expiration: ⤷ Get Started Free
Patent: 1008664
Patent: comprimidos para a terapia de combinação
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 20856
Patent: UTILISATION DE PARTICULES SUPPORTS SOLIDES POUR AMELIORER L'APTITUDE AU TRAITEMENT D'UN AGENT PHARMACEUTIQUE (THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT)
Estimated Expiration: ⤷ Get Started Free
Patent: 50521
Patent: PASTILLES DESTINEES A UNE THERAPIE COMBINEE (TABLETS FOR COMBINATION THERAPY)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 11001885
Patent: Tableta bicapa oral en donde la primera capa comprende al compuesto de formula i (elvitegravir) y al compuesto de formula ii, y la segunda capa comprende al compuesto de formula iii (emtricitabina) y a la sal de formula iv (sal de tenofovir); metodo para la preparacion, util en el tratar infecciones por vih.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2123700
Patent: The use of solid carrier particles to improve the processability of a pharmaceutical agent
Estimated Expiration: ⤷ Get Started Free
Patent: 2307573
Estimated Expiration: ⤷ Get Started Free
Patent: 3479584
Patent: Use of solid carrier particles to improve the processability of pharmaceutical agent
Estimated Expiration: ⤷ Get Started Free
Patent: 4940937
Patent: The use of solid carrier particles to improve the processability of a pharmaceutical agent
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 21225
Patent: PARTICULAS PORTADORAS SÓLIDAS PARA MEJORAR LA PROCESABILIDAD DE UN AGENTE FARMACÉUTICO
Estimated Expiration: ⤷ Get Started Free
Patent: 00187
Patent: TABLETAS PARA TERAPIA DE COMBINACIÓN
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0151009
Estimated Expiration: ⤷ Get Started Free
Patent: 0151357
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 16852
Estimated Expiration: ⤷ Get Started Free
Patent: 17067
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 96633
Estimated Expiration: ⤷ Get Started Free
Patent: 93485
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 10010636
Patent: EL USO DE PARTÍCULAS TRANSPORTADORAS SÓLIDAS PARA MEJORAR LA PROCESABILIDAD DE UN AGENTE FARMACÉUTICO
Estimated Expiration: ⤷ Get Started Free
Patent: 11011307
Patent: COMPRIMIDOS PARA TERAPIA DE COMBINACION
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 1313
Patent: ТАБЛЕТКИ ДЛЯ КОМБИНИРОВАННОЙ ТЕРАПИИ (TABLETS FOR COMBINATION THERAPY)
Estimated Expiration: ⤷ Get Started Free
Patent: 2950
Patent: ПРИМЕНЕНИЕ ЧАСТИЦ НОСИТЕЛЯ ДИОКСИДА КРЕМНИЯ ДЛЯ УЛУЧШЕНИЯ ТЕХНОЛОГИЧЕСКИХ ХАРАКТЕРИСТИК ФАРМАЦЕВТИЧЕСКОГО АГЕНТА (USE OF SILICON DIOXIDE CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT)
Estimated Expiration: ⤷ Get Started Free
Patent: 0123
Patent: ТАБЛЕТКА ДЛЯ ЛЕЧЕНИЯ ВИЧ И СПОСОБ ЛЕЧЕНИЯ ВИЧ С ЕЕ ПРИМЕНЕНИЕМ (TABLET FOR TREATING HIV AND METHOD OF TREATING HIV USING SAME)
Estimated Expiration: ⤷ Get Started Free
Patent: 1071173
Patent: ПРИМЕНЕНИЕ ЧАСТИЦ ТВЁРДОГО НОСИТЕЛЯ ДЛЯ УЛУЧШЕНИЯ ТЕХНОЛОГИЧЕСКИХ ХАРАКТЕРИСТИК ФАРМАЦЕВТИЧЕСКОГО АГЕНТА
Estimated Expiration: ⤷ Get Started Free
Patent: 1190125
Patent: ТАБЛЕТКИ ДЛЯ КОМБИНИРОВАННОЙ ТЕРАПИИ
Estimated Expiration: ⤷ Get Started Free
Patent: 1491658
Patent: ТАБЛЕТКИ ДЛЯ КОМБИНИРОВАННОЙ ТЕРАПИИ
Estimated Expiration: ⤷ Get Started Free
Patent: 1591353
Patent: ПРИМЕНЕНИЕ ЧАСТИЦ ТВЕРДОГО НОСИТЕЛЯ ДЛЯ УЛУЧШЕНИЯ ТЕХНОЛОГИЧЕСКИХ ХАРАКТЕРИСТИК ФАРМАЦЕВТИЧЕСКОГО АГЕНТА
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 96633
Patent: UTILISATION DE VEHICULES PARTICULAIRES SOLIDES POUR FACILITER LA FORMULATION D'UN AGENT PHARMACEUTIQUE. (THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT)
Estimated Expiration: ⤷ Get Started Free
Patent: 93485
Patent: COMPRIMÉ BICOUCHE CONTENANT DE L'ELVITEGRAVIR, DU COBICISTAT, DE L'EMTRICITABINE ET DU TENOFOVIR (BILAYER TABLETS COMPRISING ELVITEGRAVIR, COBICISTAT, EMTRICITABINE AND TENOFOVIR)
Estimated Expiration: ⤷ Get Started Free
Patent: 06032
Patent: UTILISATION DE PARTICULES SUPPORTS SOLIDES POUR AMÉLIORER L'APTITUDE AU TRAITEMENT D'UN AGENT PHARMACEUTIQUE (THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 53670
Patent: 利用固體載體顆粒來改進藥劑加工性 (THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT)
Estimated Expiration: ⤷ Get Started Free
Patent: 64737
Patent: 包括埃替拉韋, ,恩曲他濱和替諾福韋的雙層片劑 (BILAYER TABLETS COMPRISING ELVITEGRAVIR, COBICISTAT, EMTRICITABINE AND TENOFOVIR COBICISTAT)
Estimated Expiration: ⤷ Get Started Free
Patent: 15679
Patent: 固體載體顆粒在改善藥物製劑加工性中的應用 (THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 25822
Estimated Expiration: ⤷ Get Started Free
Patent: 26380
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 8614
Patent: שימוש בחלקיקי נשא מוצק לשיפור היכולת לעיבוד חומר רוקחי (Use of solid carrier particles to improve the processability of a pharmaceutical agent)
Estimated Expiration: ⤷ Get Started Free
Patent: 4227
Patent: טבליות עבור טיפול משולב (Tablets for combination therapy)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 11242
Estimated Expiration: ⤷ Get Started Free
Patent: 22213
Estimated Expiration: ⤷ Get Started Free
Patent: 11927
Estimated Expiration: ⤷ Get Started Free
Patent: 25171
Estimated Expiration: ⤷ Get Started Free
Patent: 11522790
Estimated Expiration: ⤷ Get Started Free
Patent: 12517432
Estimated Expiration: ⤷ Get Started Free
Patent: 14012741
Patent: USE OF SOLID CARRIER PARTICLE TO IMPROVE PROCESSABILITY OF PHARMACEUTICAL AGENT
Estimated Expiration: ⤷ Get Started Free
Patent: 14221845
Patent: 併用治療のための錠剤 (TABLETS FOR COMBINATION THERAPY)
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 2377
Patent: USO DE PARTICULAS TRASPORTADORAS SOLIDAS PARA MEJORAR LA CAPACIDAD DE PROCESAMIENTO DE UN AGENTE FARMACEUTICO. (THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT.)
Estimated Expiration: ⤷ Get Started Free
Patent: 10011963
Patent: USO DE PARTICULAS TRASPORTADORAS SOLIDAS PARA MEJORAR LA CAPACIDAD DE PROCESAMIENTO DE UN AGENTE FARMACEUTICO. (THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT.)
Estimated Expiration: ⤷ Get Started Free
Patent: 11008289
Patent: TABLETAS PARA TERAPIA COMBINADA. (TABLETS FOR COMBINATION THERAPY.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 8978
Patent: THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT
Estimated Expiration: ⤷ Get Started Free
Patent: 4214
Patent: TABLETS COMPRISING COBICISTAT, ELVITEGRAVIR, EMTRICITABINE AND TENOFOVIR DISOPROXIL FUMARATE FOR COMBINATION THERAPY
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 110994
Patent: COMPRIMIDOS ANTIVIRALES QUE COMPRENDEN ELVITEGRAVIR, EMTRICITABINA, DISOPROXIL FUMARATO DE TENOFOVIR Y UN DERIVADO DE TIAZOL
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 96633
Estimated Expiration: ⤷ Get Started Free
Patent: 93485
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 96633
Estimated Expiration: ⤷ Get Started Free
Patent: 93485
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01500266
Patent: COMPRESSE A DOPPIO STRATO COMPRENDENTI ELVITEGRAVIR, COBICISTAT, EMTRICITABINA E TENOFOVIR
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 3544
Patent: TABLETS FOR COMBINATION THERAPY
Estimated Expiration: ⤷ Get Started Free
Patent: 0618
Patent: THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT
Estimated Expiration: ⤷ Get Started Free
Patent: 14007744
Patent: TABLETS FOR COMBINATION THERAPY
Estimated Expiration: ⤷ Get Started Free
Patent: 201609006W
Patent: THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT
Estimated Expiration: ⤷ Get Started Free
Patent: 201706215U
Patent: Tablets for combination therapy
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 96633
Estimated Expiration: ⤷ Get Started Free
Patent: 93485
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1008007
Patent: THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1645759
Estimated Expiration: ⤷ Get Started Free
Patent: 1659971
Estimated Expiration: ⤷ Get Started Free
Patent: 1738325
Estimated Expiration: ⤷ Get Started Free
Patent: 1784647
Estimated Expiration: ⤷ Get Started Free
Patent: 110015581
Patent: THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT
Estimated Expiration: ⤷ Get Started Free
Patent: 110122729
Patent: TABLETS FOR COMBINATION THERAPY
Estimated Expiration: ⤷ Get Started Free
Patent: 160093100
Patent: 조합 요법용 정제 (TABLETS FOR COMBINATION THERAPY)
Estimated Expiration: ⤷ Get Started Free
Patent: 160114728
Patent: 제약 제제의 가공성 향상을 위한 고체 담체 입자의 용도 (THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 48886
Estimated Expiration: ⤷ Get Started Free
Patent: 53897
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 44367
Estimated Expiration: ⤷ Get Started Free
Patent: 1040142
Patent: Tablets for combination therapy
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 1193
Patent: ПРИМЕНЕНИЕ ЧАСТИЧЕК ТВЕРДОГО НОСИТЕЛЯ ДЛЯ УЛУЧШЕНИЯ ТЕХНОЛОГИЧЕСКИХ ХАРАКТЕРИСТИК ФАРМАЦЕВТИЧЕСКОГО АГЕНТА;ЗАСТОСУВАННЯ ЧАСТИНОК ТВЕРДОГО НОСІЯ ДЛЯ ПОЛІПШЕННЯ ТЕХНОЛОГІЧНИХ ХАРАКТЕРИСТИК ФАРМАЦЕВТИЧНОГО АГЕНТА (THE USE OF SOLID CARRIER PARTICLES TO IMPROVE THE PROCESSABILITY OF A PHARMACEUTICAL AGENT)
Estimated Expiration: ⤷ Get Started Free
Patent: 3224
Patent: ТАБЛЕТКИ ДЛЯ КОМБИНИРОВАННОЙ ТЕРАПИИ;ТАБЛЕТКИ ДЛЯ КОМБІНОВАНОЇ ТЕРАПІЇ (Normal;heading 1;heading 2;heading 3;TABLETS FOR COMBINATION THERAPY)
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 424
Patent: COMPRIMIDOS CONTENIENDO ELIVITEGRAVIR PARA TRATAMIENTO DE INFECCIONES VIRALES
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering EVOTAZ around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 101636221 | ⤷ Get Started Free | |
| Hong Kong | 1126485 | ⤷ Get Started Free | |
| Spain | 2779826 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for EVOTAZ
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2487163 | 17C1000 | France | ⤷ Get Started Free | PRODUCT NAME: COBICISTAT OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE ET ATAZANAVIR OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE,EN PARTICULIER LE SULFATE D'ATAZANAVIR; REGISTRATION NO/DATE: EU/1/15/1025 20150715 |
| 0900210 | SPC023/2005 | Ireland | ⤷ Get Started Free | SPC023/2005, 20060612, EXPIRES: 20190301 |
| 2487162 | 122016000103 | Germany | ⤷ Get Started Free | PRODUCT NAME: COBICISTAT ODER EIN PHARMAZEUTISCH AKZEPTABLES SALZ ODER SOLVAT DAVON UND DARUNAVIR ODER EIN PHARMAZEUTISCH AKZEPTABLES SALZ ODER SOLVAT DAVON; REGISTRATION NO/DATE: EU/1/14/967 20141119 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for EVOTAZ
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
