ESBRIET Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Esbriet, and when can generic versions of Esbriet launch?
Esbriet is a drug marketed by Genentech Inc and is included in two NDAs. There are twenty patents protecting this drug and two Paragraph IV challenges.
This drug has two hundred and sixty-six patent family members in forty-six countries.
The generic ingredient in ESBRIET is pirfenidone. There are twenty-three drug master file entries for this compound. Twenty-three suppliers are listed for this compound. Additional details are available on the pirfenidone profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Esbriet
A generic version of ESBRIET was approved as pirfenidone by AMNEAL on January 3rd, 2022.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ESBRIET?
- What are the global sales for ESBRIET?
- What is Average Wholesale Price for ESBRIET?
Summary for ESBRIET
| International Patents: | 266 |
| US Patents: | 20 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 103 |
| Clinical Trials: | 19 |
| Patent Applications: | 4,854 |
| Drug Prices: | Drug price information for ESBRIET |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ESBRIET |
| What excipients (inactive ingredients) are in ESBRIET? | ESBRIET excipients list |
| DailyMed Link: | ESBRIET at DailyMed |

Recent Clinical Trials for ESBRIET
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Excalibur Pharmaceuticals, Inc. | Phase 1 |
| National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) | Phase 2 |
| University of California, San Diego | Phase 2 |
Pharmacology for ESBRIET
| Drug Class | Pyridone |
Paragraph IV (Patent) Challenges for ESBRIET
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ESBRIET | Capsules | pirfenidone | 267 mg | 022535 | 9 | 2018-10-15 |
| ESBRIET | Tablets | pirfenidone | 534 mg | 208780 | 2 | 2018-10-15 |
US Patents and Regulatory Information for ESBRIET
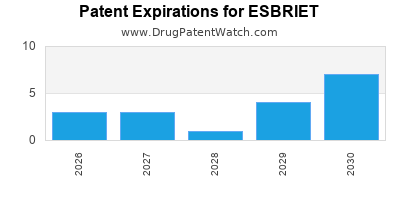
ESBRIET is protected by sixty-four US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genentech Inc | ESBRIET | pirfenidone | TABLET;ORAL | 208780-001 | Jan 11, 2017 | AB | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Genentech Inc | ESBRIET | pirfenidone | TABLET;ORAL | 208780-003 | Jan 11, 2017 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Genentech Inc | ESBRIET | pirfenidone | TABLET;ORAL | 208780-003 | Jan 11, 2017 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Genentech Inc | ESBRIET | pirfenidone | TABLET;ORAL | 208780-003 | Jan 11, 2017 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Genentech Inc | ESBRIET | pirfenidone | TABLET;ORAL | 208780-003 | Jan 11, 2017 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Genentech Inc | ESBRIET | pirfenidone | TABLET;ORAL | 208780-003 | Jan 11, 2017 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ESBRIET
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Genentech Inc | ESBRIET | pirfenidone | TABLET;ORAL | 208780-001 | Jan 11, 2017 | ⤷ Get Started Free | ⤷ Get Started Free |
| Genentech Inc | ESBRIET | pirfenidone | TABLET;ORAL | 208780-002 | Jan 11, 2017 | ⤷ Get Started Free | ⤷ Get Started Free |
| Genentech Inc | ESBRIET | pirfenidone | TABLET;ORAL | 208780-003 | Jan 11, 2017 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for ESBRIET
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Roche Registration GmbH | Esbriet | pirfenidone | EMEA/H/C/002154Esbriet is indicated in adults for the treatment of idiopathic pulmonary fibrosis. | Authorised | no | no | no | 2011-02-27 | |
| Axunio Pharma GmbH | Pirfenidone axunio (previously Pirfenidone AET) | pirfenidone | EMEA/H/C/005873Pirfenidone AET is indicated in adults for the treatment of mild to moderate idiopathic pulmonary fibrosis (IPF). | Authorised | yes | no | no | 2022-06-20 | |
| Viatris Limited | Pirfenidone Viatris | pirfenidone | EMEA/H/C/005862Pirfenidone Viatris is indicated in adults for the treatment of mild to moderate idiopathic pulmonary fibrosis (IPF). | Authorised | yes | no | no | 2023-01-10 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ESBRIET
When does loss-of-exclusivity occur for ESBRIET?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 55
Estimated Expiration: ⤷ Get Started Free
Argentina
Patent: 7990
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 11201520
Estimated Expiration: ⤷ Get Started Free
Patent: 13201986
Estimated Expiration: ⤷ Get Started Free
Patent: 14240300
Estimated Expiration: ⤷ Get Started Free
Patent: 17241530
Estimated Expiration: ⤷ Get Started Free
Patent: 22275529
Patent: Granulate formulation of 5-methyl-1-phenyl-2(1H)-pyridone and method of making the same
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0616324
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 20380
Estimated Expiration: ⤷ Get Started Free
Patent: 37365
Patent: FORMULATION EN GRANULES DE 5-METHYL-1-PHENYL-2-(1H)-PYRIDONE ET METHODE DE FABRICATION ASSOCIEE (GRANULATE FORMULATION OF 5-METHYL-1-PHENYL-2-(1H)-PYRIDONE AND METHOD OF MAKING THE SAME)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1267810
Estimated Expiration: ⤷ Get Started Free
Patent: 3393607
Estimated Expiration: ⤷ Get Started Free
Patent: 3735530
Estimated Expiration: ⤷ Get Started Free
Patent: 8883072
Patent: 5-甲基-1-苯基-2-(1H)-吡啶酮颗粒制剂和其制备方法 (GRANULATE FORMULATION OF 5-METHY-1-PHENY-2(1H)-PYRIDONE AND METHOD OF MAKING THE SAME)
Estimated Expiration: ⤷ Get Started Free
Patent: 4533688
Patent: 5-甲基-1-苯基-2-(1H)-吡啶酮颗粒制剂和其制备方法 (5-methyl-1-phenyl-2-(1H)-pyridone granular formulations and processes for their preparation)
Estimated Expiration: ⤷ Get Started Free
Cuba
Patent: 080043
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 15544
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 40364
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 088394
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 0800881
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 40364
Estimated Expiration: ⤷ Get Started Free
Patent: 31025
Estimated Expiration: ⤷ Get Started Free
Patent: 35985
Patent: PRÉPARATION DE GRANULÉS DE 5-MÉTHYL-1-PHÉNYL-2(1H)-PYRIDONE ET SON PROCÉDÉ DE FABRICATION (GRANULATE FORMULATION OF 5-METHY!-1-PHENY!-2(1H)-PYRIDONE AND METHOD OF MAKING THE SAME)
Estimated Expiration: ⤷ Get Started Free
Patent: 95696
Patent: PRÉPARATION DE GRANULÉS DE 5-MÉTHYL-1-PHÉNYL-2(1H)-PYRIDONE ET SON PROCÉDÉ DE FABRICATION (GRANULATE FORMULATION OF 5-METHYL-1-PHENYL-2(1H)-PYRIDONE AND METHOD OF MAKING THE SAME)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 17762
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 9273
Estimated Expiration: ⤷ Get Started Free
Patent: 1745
Patent: פורמולציית גרגירים של 5-מתיל-1-פניל-2(h1)-פירידון ושיטה להכנתה (Granulate formulation of 5-methyl-1-phenyl-2(1h)-pyridone and method of making the same)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 15101
Estimated Expiration: ⤷ Get Started Free
Patent: 37732
Estimated Expiration: ⤷ Get Started Free
Patent: 56721
Estimated Expiration: ⤷ Get Started Free
Patent: 09509962
Estimated Expiration: ⤷ Get Started Free
Patent: 19513145
Patent: 5−メチル−1−フェニル−2−(1H)−ピリドンの顆粒製剤及びその製造方法
Estimated Expiration: ⤷ Get Started Free
Patent: 22087115
Patent: 5-メチル-1-フェニル-2-(1H)-ピリドンの顆粒製剤及びその製造方法
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 3177
Patent: FORMULACION GRANULADA DE 5-METIL-1-FENIL-2-(1H)-PIRIDONA Y METODO PARA ELABORARLA. (GRANULATE FORMULATION OF 5-METHY|-1-PHENY|-2(1H)-PYRIDONE AND METHOD OF MAKING THE SAME)
Estimated Expiration: ⤷ Get Started Free
Patent: 08003882
Estimated Expiration: ⤷ Get Started Free
Patent: 18011819
Patent: FORMULACION GRANULADA DE 5-METIL-1-FENIL-2-(1H)-PIRIDONA Y METODO PARA ELABORARLA. (GRANULATE FORMULATION OF 5-METHY|-1-PHENY|-2(1H)-PYRIDONE AND METHOD OF MAKING THE SAME.)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 875
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 5957
Estimated Expiration: ⤷ Get Started Free
Patent: 0129
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 5131
Estimated Expiration: ⤷ Get Started Free
Patent: 080759
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 40364
Estimated Expiration: ⤷ Get Started Free
Patent: 35985
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 40364
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0802237
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1675651
Estimated Expiration: ⤷ Get Started Free
Patent: 2552615
Estimated Expiration: ⤷ Get Started Free
Patent: 130100381
Estimated Expiration: ⤷ Get Started Free
Patent: 180123067
Patent: 5-메틸-1-페닐-2-(1H)-피리돈의 과립화 제형 및 이의 제조 방법
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 83595
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 5861
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ESBRIET around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Africa | 201102411 | PIRFENIDONE TREATMENT FOR PATIENTS WITH ATYPICAL LIVER FUNCTION | ⤷ Get Started Free |
| Australia | 2011201520 | ⤷ Get Started Free | |
| Portugal | 1940364 | ⤷ Get Started Free | |
| European Patent Office | 2343070 | ⤷ Get Started Free | |
| Croatia | P20110478 | ⤷ Get Started Free | |
| European Patent Office | 2308491 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for ESBRIET (Esbrerit)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
