DUZALLO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Duzallo, and what generic alternatives are available?
Duzallo is a drug marketed by Ironwood Pharms Inc and is included in one NDA. There are nine patents protecting this drug.
This drug has two hundred and one patent family members in forty-three countries.
The generic ingredient in DUZALLO is allopurinol; lesinurad. There are twenty-two drug master file entries for this compound. Additional details are available on the allopurinol; lesinurad profile page.
DrugPatentWatch® Generic Entry Outlook for Duzallo
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be February 29, 2032. This may change due to patent challenges or generic licensing.
There have been five patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for DUZALLO?
- What are the global sales for DUZALLO?
- What is Average Wholesale Price for DUZALLO?
Summary for DUZALLO
| International Patents: | 201 |
| US Patents: | 9 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Drug Prices: | Drug price information for DUZALLO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for DUZALLO |
| DailyMed Link: | DUZALLO at DailyMed |
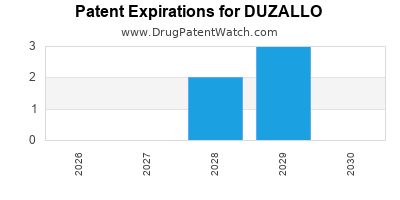

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for DUZALLO
Generic Entry Date for DUZALLO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
US Patents and Regulatory Information for DUZALLO
DUZALLO is protected by nine US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of DUZALLO is ⤷ Get Started Free.
This potential generic entry date is based on patent 8,546,436.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ironwood Pharms Inc | DUZALLO | allopurinol; lesinurad | TABLET;ORAL | 209203-001 | Aug 18, 2017 | DISCN | Yes | No | 9,216,179 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Ironwood Pharms Inc | DUZALLO | allopurinol; lesinurad | TABLET;ORAL | 209203-002 | Aug 18, 2017 | DISCN | Yes | No | 8,546,436 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Ironwood Pharms Inc | DUZALLO | allopurinol; lesinurad | TABLET;ORAL | 209203-001 | Aug 18, 2017 | DISCN | Yes | No | 10,183,012 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Ironwood Pharms Inc | DUZALLO | allopurinol; lesinurad | TABLET;ORAL | 209203-001 | Aug 18, 2017 | DISCN | Yes | No | 8,283,369 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for DUZALLO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Ironwood Pharms Inc | DUZALLO | allopurinol; lesinurad | TABLET;ORAL | 209203-002 | Aug 18, 2017 | 8,003,681 | ⤷ Get Started Free |
| Ironwood Pharms Inc | DUZALLO | allopurinol; lesinurad | TABLET;ORAL | 209203-001 | Aug 18, 2017 | 8,003,681 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for DUZALLO
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Grunenthal GmbH | Duzallo | allopurinol, lesinurad | EMEA/H/C/004412Duzallo is indicated in adults for the treatment of hyperuricaemia in gout patients who have not achieved target serum uric acid levels with an adequate dose of allopurinol alone. | Withdrawn | no | no | no | 2018-08-23 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for DUZALLO
When does loss-of-exclusivity occur for DUZALLO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 4639
Patent: FORMAS POLIMORFICAS DE ACIDO 2-(5-BROMO-4-(4-CICLOPROPILNAFTALEN-1-IL)-4H-1,2,4-TRIAZOL-3-ILTIO)ACETICO Y USOS DEL MISMO
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 11352129
Patent: Polymorphic forms of 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-3ylthio)acetic acid and uses thereof
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2013016982
Patent: formas polimórficas de ácido 2-(5-bromo-4-(4-ciclopropilnaftalen-1-il)-4h-1,2,4-triazol-3-iltio)acético e usos destes
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 17249
Patent: FORMES POLYMORPHES DE L'ACIDE 2-(5-BROMO-4-(4-CYCLOPROPYLNAPHTALEN-1-YL)-4H-1,2,4-TRIAZOL-3-YLTHIO) ACETIQUE ET LEURS UTILISATIONS (POLYMORPHIC FORMS OF 2-(5-BROMO-4-(4-CYCLOPROPYLNAPHTHALEN-1-YL)-4H-1,2,4-TRIAZOL-3-YLTHIO) ACETIC ACID AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 3298796
Patent: Polymorphic forms of 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio) acetic acid and uses thereof
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0170187
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 18621
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 58846
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 2301
Patent: ПОЛИМОРФНЫЕ ФОРМЫ 2-(5-БРОМ-4-(4-ЦИКЛОПРОПИЛНАФТАЛИН-1-ИЛ)-4H-1,2,4-ТРИАЗОЛ-3-ИЛТИО)УКСУСНОЙ КИСЛОТЫ И ИХ ПРИМЕНЕНИЕ (POLYMORPHIC FORMS OF 2-(5-BROMO-4-(4-CYCLOPROPYLNAPHTHALEN-1-YL)-4H-1,2,4-TRIAZOL-3-YLTHIO)ACETIC ACID AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 1370150
Patent: ПОЛИМОРФНЫЕ ФОРМЫ 2-(5-БРОМ-4-(4-ЦИКЛОПРОПИЛНАФТАЛИН-1-ИЛ)-4H-1,2,4-ТРИАЗОЛ-3-ИЛТИО)УКСУСНОЙ КИСЛОТЫ И ИХ ПРИМЕНЕНИЕ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 58846
Patent: FORMES POLYMORPHES DE L'ACIDE 2-(5-BROMO-4-(4-CYCLOPROPYLNAPHTALEN-1-YL)-4H-1,2,4-TRIAZOL-3-YLTHIO) ACÉTIQUE ET LEURS UTILISATIONS (POLYMORPHIC FORMS OF 2-(5-BROMO-4-(4-CYCLOPROPYLNAPHTHALEN-1-YL)-4H-1,2,4-TRIAZOL-3-YLTHIO) ACETIC ACID AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 31766
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 6367
Patent: צורות פולימורפיות גבישיות של 2-(5-ברומו-4-(4-ציקלופרופילנפתלן -1-איל)-4h-4,2,1-טריאזול-3-אילתיאו) חומצה אצטית, תכשירי רוקחות מוצקים המכילים אותן ושיטות להכנתן (Polymorphic forms of crystalline polymorph of 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)4h-1,2,4-triazol-3-ylthio) acetic acid, solid pharmaceutical compositions comprising them and a process for their preparation)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 81627
Estimated Expiration: ⤷ Get Started Free
Patent: 14501282
Estimated Expiration: ⤷ Get Started Free
Patent: 15172053
Patent: 2−(5−ブロモ−4−(4−シクロプロピルナフタレン−1−イル)−4H−1,2,4−トリアゾル−3−イルチオ)酢酸の多形形態およびその使用 (POLYMORPHIC FORMS OF 2-(5-BROMO-4-(4-CYCLOPROPYLNAPHTHALEN-1-YL)-4H-1,2,4-TRIAZOL-3-YLTHIO)ACETIC ACID AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 58846
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 2534
Patent: POLYMORPHIC FORMS OF 2-(5-BROMO-4-(4-CYCLOPROPYLNAPHTHALEN-1-YL)-4H-1,2,4-TRIAZOL-3-YLTHIO)ACETIC ACID AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 13007505
Patent: FORMAS POLIMORFICAS DE ACIDO 2-(5-BROMO-4-(4-CICLOPROPILNAFTALEN-1 -IL)-4H-1,2,4-TRIAZOL-3-ILTIO)ACETICO Y USOS DE LOS MISMOS. (POLYMORPHIC FORMS OF 2-(5-BROMO-4-(4-CYCLOPROPYLNAPHTHALEN-1-YL)- 4H-1,2,4-TRIAZOL-3-YLTHIO) ACETIC ACID AND USES THEREOF.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 0104
Patent: Polymorphic forms of 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4h-1,2,4-triazol-3-ylthio) acetic acid and uses thereof
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 58846
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 58846
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 667
Patent: POLIMORFNE FORME 2-(5-BROMO-4-(4-CILKOPROPILNAFTALEN-1-IL)-4H-1,2,4-TRIAZOL-3-ILTIO) SIRĆETNE KISELINE I NJIHOVA UPOTREBA (POLYMORPHIC FORMS OF 2-(5-BROMO-4-(4-CYCLOPROPYLNAPHTHALEN-1-YL)-4H-1,2,4-TRIAZOL-3-YLTHIO) ACETIC ACID AND USES THEREOF)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 0902
Patent: POLYMORPHIC FORMS OF 2-(5-BROMO-4-(4-CYCLOPROPYLNAPHTHALEN-1-YL)-4H-1,2,4-TRIAZOL-3-YLTHIO) ACETIC ACID AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 58846
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1303253
Patent: POLYMORPHIC FORMS OF 2-(5-BROMO-4-(4-CYCLOPROPYLNAPHTHALEN-1-YL)-4H-1,2,4-TRIAZOL-3-YL-THIO)ACETIC ACID AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1541629
Estimated Expiration: ⤷ Get Started Free
Patent: 130105902
Patent: POLYMORPHIC FORMS OF 2-(5-BROMO-4-(4-CYCLOPROPYLNAPHTHALEN-1-YL)-4H-1,2,4-TRIAZOL-3-YLTHIO)ACETIC ACID AND USES THEREOF
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 14914
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 52037
Estimated Expiration: ⤷ Get Started Free
Patent: 1302718
Patent: Polymorphic forms of 2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid and uses thereof
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 9172
Patent: ПОЛІМОРФНА ФОРМА 2-(5-БРОМ-4-(4-ЦИКЛОПРОПІЛНАФТАЛІН-1-ІЛ)-4H-1,2,4-ТРИАЗОЛ-3-ІЛТІО)ОЦТОВОЇ КИСЛОТИ (ВАРІАНТИ) ТА ЇЇ ЗАСТОСУВАННЯ
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering DUZALLO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Israel | 201546 | 2-(5-ברומו-4-(4-ציקלופרופילנפתלן-1-איל)-h4- 1, 2, 4-טריאזול-3-תיאו) חומצה אצטית ומתיל האסטר (2-(5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4h-1,2,4-triazol-3-ylthio)acetic acid and methyl ester thereof) | ⤷ Get Started Free |
| Japan | 2015172053 | ⤷ Get Started Free | |
| Canada | 2736117 | ⤷ Get Started Free | |
| South Korea | 20090111357 | S-TRIAZOLYL ?-MERCAPTOACETANILIDES AS INHIBITORS OF HIV REVERSE TRANSCRIPTASE | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for DUZALLO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2135608 | C 2016 027 | Romania | ⤷ Get Started Free | PRODUCT NAME: LESINURAD SAU O SARE ACCEPTABILA FARMACEUTIC AACESTUIA; NATIONAL AUTHORISATION NUMBER: EU/1/15/1080; DATE OF NATIONAL AUTHORISATION: 20160218; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/15/1080; DATE OF FIRST AUTHORISATION IN EEA: 20160218 |
| 2217577 | LUC00103 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: DUZALLO - ALLOPURINOL/LESINURAD OU UN/DES SEL(S) PHARMACEUTIQUEMENT ACCEPTABLE(S) DE CELUI-CI; AUTHORISATION NUMBER AND DATE: EU/1/18/1300 20180827 |
| 2217577 | 122019000008 | Germany | ⤷ Get Started Free | PRODUCT NAME: DUZALLO - ALLOPURINOL / LESINURAD ODER EIN PHARMAZEUTISCH VERTRAEGLICHES SALZ ODER VERTRAEGLICHE SALZE DAVON; REGISTRATION NO/DATE: EU/1/18/1300 20180823 |
| 2217577 | 2019C/502 | Belgium | ⤷ Get Started Free | PRODUCT NAME: DUZALLO - ALLOPURINOL / LESINURAD OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE OU UN DE SES SELS; AUTHORISATION NUMBER AND DATE: EU/1/18/1300 20180827 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for DUZALLO
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
