ZYKADIA Drug Patent Profile
✉ Email this page to a colleague
When do Zykadia patents expire, and when can generic versions of Zykadia launch?
Zykadia is a drug marketed by Novartis and is included in two NDAs. There are eight patents protecting this drug.
This drug has three hundred and twenty-two patent family members in fifty-six countries.
The generic ingredient in ZYKADIA is ceritinib. There is one drug master file entry for this compound. One supplier is listed for this compound. Additional details are available on the ceritinib profile page.
DrugPatentWatch® Generic Entry Outlook for Zykadia
Zykadia was eligible for patent challenges on April 29, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be January 18, 2032. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ZYKADIA?
- What are the global sales for ZYKADIA?
- What is Average Wholesale Price for ZYKADIA?
Summary for ZYKADIA
| International Patents: | 322 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 78 |
| Clinical Trials: | 11 |
| Patent Applications: | 4,090 |
| Drug Prices: | Drug price information for ZYKADIA |
| What excipients (inactive ingredients) are in ZYKADIA? | ZYKADIA excipients list |
| DailyMed Link: | ZYKADIA at DailyMed |
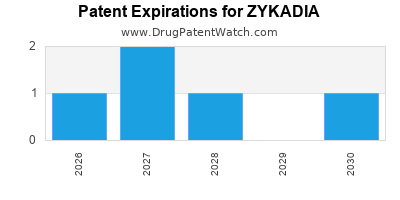

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ZYKADIA
Generic Entry Dates for ZYKADIA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
Generic Entry Dates for ZYKADIA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ZYKADIA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Commissariat A L'energie Atomique | Phase 3 |
| Plateforme labellisée Inca – Hôpital Européen Georges Pompidou, Paris | Phase 3 |
| Institut Bergonié | Phase 3 |
Pharmacology for ZYKADIA
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Cytochrome P450 2C9 Inhibitors Cytochrome P450 3A Inhibitors Tyrosine Kinase Inhibitors |
US Patents and Regulatory Information for ZYKADIA
ZYKADIA is protected by eight US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ZYKADIA is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | ZYKADIA | ceritinib | CAPSULE;ORAL | 205755-001 | Apr 29, 2014 | DISCN | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Novartis | ZYKADIA | ceritinib | CAPSULE;ORAL | 205755-001 | Apr 29, 2014 | DISCN | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Novartis | ZYKADIA | ceritinib | CAPSULE;ORAL | 205755-001 | Apr 29, 2014 | DISCN | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Novartis | ZYKADIA | ceritinib | TABLET;ORAL | 211225-001 | Mar 18, 2019 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Novartis | ZYKADIA | ceritinib | TABLET;ORAL | 211225-001 | Mar 18, 2019 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ZYKADIA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novartis | ZYKADIA | ceritinib | TABLET;ORAL | 211225-001 | Mar 18, 2019 | ⤷ Get Started Free | ⤷ Get Started Free |
| Novartis | ZYKADIA | ceritinib | CAPSULE;ORAL | 205755-001 | Apr 29, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| Novartis | ZYKADIA | ceritinib | CAPSULE;ORAL | 205755-001 | Apr 29, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| Novartis | ZYKADIA | ceritinib | TABLET;ORAL | 211225-001 | Mar 18, 2019 | ⤷ Get Started Free | ⤷ Get Started Free |
| Novartis | ZYKADIA | ceritinib | CAPSULE;ORAL | 205755-001 | Apr 29, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for ZYKADIA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Novartis Europharm Limited | Zykadia | ceritinib | EMEA/H/C/003819Zykadia is indicated for the treatment of adult patients with anaplastic lymphoma kinase (ALK) positive advanced non small cell lung cancer (NSCLC) previously treated with crizotinib. | Authorised | no | no | no | 2015-05-06 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ZYKADIA
When does loss-of-exclusivity occur for ZYKADIA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 4309
Estimated Expiration: ⤷ Get Started Free
Patent: 2395
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 11343775
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2013015000
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 21102
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 13001723
Estimated Expiration: ⤷ Get Started Free
China
Patent: 3282359
Estimated Expiration: ⤷ Get Started Free
Patent: 4262324
Estimated Expiration: ⤷ Get Started Free
Patent: 6008462
Estimated Expiration: ⤷ Get Started Free
Patent: 6831716
Estimated Expiration: ⤷ Get Started Free
Patent: 7056751
Estimated Expiration: ⤷ Get Started Free
Patent: 2125884
Estimated Expiration: ⤷ Get Started Free
Patent: 4989139
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 01792
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0171477
Estimated Expiration: ⤷ Get Started Free
Patent: 0181737
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 19474
Estimated Expiration: ⤷ Get Started Free
Patent: 21017
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 51918
Estimated Expiration: ⤷ Get Started Free
Patent: 21171
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 13012770
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 51918
Estimated Expiration: ⤷ Get Started Free
Patent: 21171
Estimated Expiration: ⤷ Get Started Free
Patent: 53708
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 1300153
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 41845
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 6474
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 16752
Estimated Expiration: ⤷ Get Started Free
Patent: 13545812
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 51918
Estimated Expiration: ⤷ Get Started Free
Patent: 21171
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 4810
Estimated Expiration: ⤷ Get Started Free
Patent: 7742
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 8210
Estimated Expiration: ⤷ Get Started Free
Patent: 13006952
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 771
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 0713
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 140698
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 013501254
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 51918
Estimated Expiration: ⤷ Get Started Free
Patent: 21171
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 51918
Estimated Expiration: ⤷ Get Started Free
Patent: 21171
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 99785
Patent: КРИСТАЛЛИЧЕСКИЕ ФОРМЫ 5-ХЛОР-N2-(2-ИЗОПРОПОКСИ-5-МЕТИЛ-4-ПИПЕРИДИН-4-ИЛ-ФЕНИЛ)-N4-[2-(ПРОПАН-2-СУЛЬФОНИЛ)-ФЕНИЛ]-ПИРИМИДИН-2,4-ДИАМИНА (CRYSTALLINE FORMS OF 5-CHLORO-N2-(2-ISOPROPOXY-5-METHYL-4-PIPERIDIN-4-YL-PHENYL)-N4-[2-(PROPANE-2-SULPHONYL)-PHENYL]-PYRIMIDINE-2,4-DIAMINE)
Estimated Expiration: ⤷ Get Started Free
Patent: 46159
Patent: КРИСТАЛЛИЧЕСКИЕ ФОРМЫ 5-ХЛОР-N2-(2-ИЗОПРОПОКСИ-5-МЕТИЛ-4-ПИПЕРИДИН-4-ИЛ-ФЕНИЛ)-N4-[2-(ПРОПАН-2-СУЛЬФОНИЛ)-ФЕНИЛ]-ПИРИМИДИН-2,4-ДИАМИНА (CRYSTAL FORMS 5-CHLORO-N2 - (2-ISOPROPOXY-5-METHYL-4-PIPERIDINE-4-YL-PHENYL) - N 4 - [2-(PROPANE-2-SULFONYL)- PHENYL] - PYRIMIDINE-2,4-DIAMINE)
Estimated Expiration: ⤷ Get Started Free
Patent: 13132947
Patent: КРИСТАЛЛИЧЕСКИЕ ФОРМЫ 5-ХЛОР-N2-(2-ИЗОПРОПОКСИ-5-МЕТИЛ-4-ПИПЕРИДИН-4-ИЛ-ФЕНИЛ)-N4-[2-(ПРОПАН-2-СУЛЬФОНИЛ)-ФЕНИЛ]-ПИРИМИДИН-2,4-ДИАМИНА
Estimated Expiration: ⤷ Get Started Free
Patent: 16136823
Patent: КРИСТАЛЛИЧЕСКИЕ ФОРМЫ 5-ХЛОР-N2-(2-ИЗОПРОПОКСИ-5-МЕТИЛ-4-ПИПЕРИДИН-4-ИЛ-ФЕНИЛ)-N4-[2-(ПРОПАН-2-СУЛЬФОНИЛ)-ФЕНИЛ]-ПИРИМИДИН-2,4-ДИАМИНА
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 771
Patent: KRISTALNI OBLICI 5-HLORO-N2-(2-IZOPROPOKSI-5-METIL-4-PIPERIDIN-4-IL-FENIL)-N4-[2-(PROPAN-2-SULFONIL)-FENIL]-PIRIMIDIN-2,4-DIAMINA (CRYSTALLINE FORMS OF 5-CHLORO-N2-(2-ISOPROPOXY-5-METHYL-4-PIPERIDIN-4-YL-PHENYL)-N4[2-(PROPANE-2-SULFONYL)-PHENYL]-PYRIMIDINE-2,4-DIAMINE)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 0856
Patent: CRYSTALLINE FORMS OF 5-CHLORO-N2-(2-ISOPROPOXY-5-METHYL-4-PIPERIDIN-4-YL-PHENYL)-N4[2-(PROPANE-2-SULFONYL)-PHENYL]-PYRIMIDINE-2,4-DIAMINE
Estimated Expiration: ⤷ Get Started Free
Patent: 201510082X
Patent: CRYSTALLINE FORMS OF 5-CHLORO-N2-(2-ISOPROPOXY-5-METHYL-4-PIPERIDIN-4-YL-PHENYL)-N4[2-(PROPANE-2-SULFONYL)-PHENYL]-PYRIMIDINE-2,4-DIAMINE
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 51918
Estimated Expiration: ⤷ Get Started Free
Patent: 21171
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1303599
Patent: CRYSTALLINE FORMS OF 5-CHLORO-N2-(2-METHYL-4-PIPERIDIN-4-YL-PHENYL)-N4[2-(PROPANE-2-SULFONYL)-PHENYL]-PYRIMIDINE2-,4-DIAMINE
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2325775
Estimated Expiration: ⤷ Get Started Free
Patent: 130130022
Estimated Expiration: ⤷ Get Started Free
Patent: 180032680
Estimated Expiration: ⤷ Get Started Free
Patent: 190022903
Estimated Expiration: ⤷ Get Started Free
Patent: 200039021
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 43016
Estimated Expiration: ⤷ Get Started Free
Patent: 96526
Estimated Expiration: ⤷ Get Started Free
Patent: 05973
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 76343
Estimated Expiration: ⤷ Get Started Free
Patent: 76344
Estimated Expiration: ⤷ Get Started Free
Patent: 1307299
Patent: Crystalline forms of 5-chloro-N2-(2-isopropoxy-5-methyl-4-piperidin-4-yl-phenyl)-N4-[2-(propane-2-sulfonyl)-phenyl]-pyrimidine-2,4-diamine
Estimated Expiration: ⤷ Get Started Free
Patent: 1629021
Patent: Crystalline forms of 5-chloro-N2-(2-isopropoxy-5-methyl-4-piperidin-4-yl-phenyl)-N4-[2-(propane-2-sulfonyl)-phenyl]-pyrimidine-2,4-diamine
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 13000216
Patent: CRYSTALLINE FORMS OF 5-CHLORO-N2-(2-ISOPROPOXY-5-METHYL-4-PIPERIDIN-4-YL-PHENYL)-N4[2-(PROPANE-2-SULFONYL)-PHENYL]-PYRIMIDINE-2,4-DIAMINE
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ZYKADIA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20090087127 | ⤷ Get Started Free | |
| South Korea | 20190022903 | ⤷ Get Started Free | |
| Russian Federation | 2376992 | СПОСОБЫ ЛЕЧЕНИЯ ИЛИ ПРОФИЛАКТИКИ АУТОИММУННЫХ ЗАБОЛЕВАНИЙ С ПОМОЩЬЮ СОЕДИНЕНИЙ 2,4-ПИРИМИДИНДИАМИНА (METHODS OF TREATMENT OR PREVENTION OF AUTOIMMUNE DISEASES BY MEANS OF 2,4-PYRIMIDINDIAMIN COMPOUNDS) | ⤷ Get Started Free |
| Iceland | 8349 | 2,4-pýrimídíndíamín gagnleg í meðhöndlun æxlissjúkdóma, bólgu- og ónæmiskerfissjúkdóma | ⤷ Get Started Free |
| Poland | 373530 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ZYKADIA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2091918 | 132016000025233 | Italy | ⤷ Get Started Free | PRODUCT NAME: CERITINIB O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE(ZYKADIA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/15/999/001, 20150508 |
| 2091918 | 300763 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: CERITINIB, OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/15/999 20150508 |
| 2091918 | 92785 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: CERITINIB OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI. FIRST REGISTRATION: 20150508 |
| 2091918 | 528 | Finland | ⤷ Get Started Free | |
| 1272477 | 529 | Finland | ⤷ Get Started Free | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for ZYKADIA (Crizotinib)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
