TRELSTAR Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Trelstar, and when can generic versions of Trelstar launch?
Trelstar is a drug marketed by Verity and is included in three NDAs. There is one patent protecting this drug.
This drug has forty-seven patent family members in thirty-two countries.
The generic ingredient in TRELSTAR is triptorelin pamoate. There are three drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the triptorelin pamoate profile page.
DrugPatentWatch® Generic Entry Outlook for Trelstar
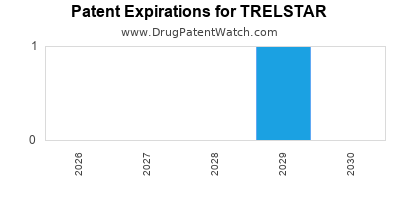
Trelstar was eligible for patent challenges on June 15, 2004.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 30, 2029. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TRELSTAR?
- What are the global sales for TRELSTAR?
- What is Average Wholesale Price for TRELSTAR?
Summary for TRELSTAR
| International Patents: | 47 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 3 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 12 |
| Patent Applications: | 1,149 |
| Drug Prices: | Drug price information for TRELSTAR |
| What excipients (inactive ingredients) are in TRELSTAR? | TRELSTAR excipients list |
| DailyMed Link: | TRELSTAR at DailyMed |

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TRELSTAR
Generic Entry Date for TRELSTAR*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
INJECTABLE;INTRAMUSCULAR |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for TRELSTAR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Zenith Epigenetics | PHASE2 |
| National Cancer Institute (NCI) | PHASE2 |
| Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins | PHASE2 |
US Patents and Regulatory Information for TRELSTAR
TRELSTAR is protected by one US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TRELSTAR is ⤷ Get Started Free.
This potential generic entry date is based on patent 10,166,181.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Verity | TRELSTAR | triptorelin pamoate | INJECTABLE;INTRAMUSCULAR | 020715-001 | Jun 15, 2000 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Verity | TRELSTAR | triptorelin pamoate | INJECTABLE;INTRAMUSCULAR | 021288-001 | Jun 29, 2001 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Verity | TRELSTAR | triptorelin pamoate | INJECTABLE;INTRAMUSCULAR | 022437-001 | Mar 10, 2010 | RX | Yes | Yes | 10,166,181 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TRELSTAR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Verity | TRELSTAR | triptorelin pamoate | INJECTABLE;INTRAMUSCULAR | 022437-001 | Mar 10, 2010 | 5,776,885 | ⤷ Get Started Free |
| Verity | TRELSTAR | triptorelin pamoate | INJECTABLE;INTRAMUSCULAR | 020715-001 | Jun 15, 2000 | 5,134,122 | ⤷ Get Started Free |
| Verity | TRELSTAR | triptorelin pamoate | INJECTABLE;INTRAMUSCULAR | 021288-001 | Jun 29, 2001 | 5,192,741 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TRELSTAR
When does loss-of-exclusivity occur for TRELSTAR?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 00
Patent: Slow release pharmaceutical composition made of microparticles
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 08259411
Patent: Slow release pharmaceutical composition made of microparticles
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0812250
Patent: COMPOSIÇÃO FARMACÊUTICA DE LIBERAÇÃO LENTA FEITA DE MICROPARTÍCULAS
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 88478
Patent: COMPOSITION PHARMACEUTIQUE A LIBERATION LENTE, FAITE DE MICROPARTICULES (SLOW RELEASE PHARMACEUTICAL COMPOSITION MADE OF MICROPARTICLES)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1677959
Patent: Slow release pharmaceutical composition made of microparticles
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 51234
Patent: COMPOSICIONES QUE COMPRENDEN MICROPARTICULAS DE UN COPOLIMERO DE ACIDO LACTICO Y GLICOLICO (PLGA) CON UNA SUSTANCIA ACTIVA EN LA FORMA DE UNA SAL PEPTIDICA INSOLUBLE
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0161785
Estimated Expiration: ⤷ Get Started Free
Patent: 0181854
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 18434
Estimated Expiration: ⤷ Get Started Free
Patent: 20891
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 64467
Estimated Expiration: ⤷ Get Started Free
Patent: 00014
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 9284
Patent: ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ С ПРОЛОНГИРОВАННЫМ ВЫСВОБОЖДЕНИЕМ, ПРИГОТОВЛЕННАЯ ИЗ МИКРОЧАСТИЦ (SLOW RELEASE PHARMACEUTICAL COMPOSITION MADE OF MICROPARTICLES)
Estimated Expiration: ⤷ Get Started Free
Patent: 0971132
Patent: ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ С ПРОЛОНГИРОВАННЫМ ВЫСВОБОЖДЕНИЕМ, ПРИГОТОВЛЕННАЯ ИЗ МИКРОЧАСТИЦ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 64467
Patent: COMPOSITION PHARMACEUTIQUE À LIBÉRATION LENTE, FAITE DE MICROPARTICULES (SLOW RELEASE PHARMACEUTICAL COMPOSITION MADE OF MICROPARTICLES)
Estimated Expiration: ⤷ Get Started Free
Patent: 00014
Patent: Composition pharmaceutique à libération lente, faite de microparticules (Slow release pharmaceutical composition made of microparticles)
Estimated Expiration: ⤷ Get Started Free
Patent: 31077
Patent: COMPOSITION PHARMACEUTIQUE À LIBÉRATION LENTE FABRIQUÉE À PARTIR DE MICROPARTICULES (SLOW RELEASE PHARMACEUTICAL COMPOSITION MADE OF MICROPARTICLES)
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 00014
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 41737
Patent: SLOW RELEASE PHARMACEUTICAL COMPOSITION MADE OF MICROPARTICLES
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 31550
Estimated Expiration: ⤷ Get Started Free
Patent: 40391
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 2501
Patent: תכשירים המכילים מיקרו חלקיקים לשחרור מבוקר של טריפטולרין ושימוש בהם להכנת תרופה לטיפול בסרטן הערמונית (Pharmaceutical composition made of microparticles for controlled release of triptorelin and use thereof for the manufacture of a drug for the treatment of prostate cancer)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 19169
Estimated Expiration: ⤷ Get Started Free
Patent: 10529106
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 64467
Estimated Expiration: ⤷ Get Started Free
Patent: 00014
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 0450
Patent: SLOW RELEASE PHARMACEUTICAL COMPOSITION MADE OF MICROPARTICLES
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 09012856
Patent: COMPOSICION FARMACEUTICA DE LIBERACION LENTA HECHA DE MICROPARTICULAS. (SLOW RELEASE PHARMACEUTICAL COMPOSITION MADE OF MICROPARTICLES.)
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 959
Patent: FARMACEUTSKA KOMPOZICIJA SA SPORIM OSLOBAĐANJEM KOJA JE FORMIRANA OD MIKROČESTICA (SLOW RELEASE PHARMACEUTICAL COMPOSITION MADE OF MICROPARTICLES)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 422
Patent: COMPOSITION PHARMACEUTIQUE A LIBERATION PROLONGEE CONSTITUEE DE MICROPARTICULES
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 2423
Patent: SLOW RELEASE PHARMACEUTICAL COMPOSITION MADE OF MICROPARTICLES COMPRISING PLGA, LHRH AND LACTIC ACID
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 64467
Estimated Expiration: ⤷ Get Started Free
Patent: 00014
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 64467
Estimated Expiration: ⤷ Get Started Free
Patent: 00014
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 591
Patent: FARMACEUTSKA KOMPOZICIJA SA SPORIM OSLOBAĐANJEM PRIPREMLJENA OD MIKROČESTICA (SLOW RELEASE PHARMACEUTICAL COMPOSITION MADE OF MICROPARTICLES)
Estimated Expiration: ⤷ Get Started Free
Patent: 248
Patent: FARMACEUTSKA KOMPOZICIJA SA SPORIM OSLOBAĐANJEM PRIPREMLJENA OD MIKROČESTICA (SLOW RELEASE PHARMACEUTICAL COMPOSITION MADE OF MICROPARTICLES)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 64467
Estimated Expiration: ⤷ Get Started Free
Patent: 00014
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0907940
Patent: Slow release pharmaceutical composition made of microparticles
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1631475
Estimated Expiration: ⤷ Get Started Free
Patent: 100023950
Patent: SLOW RELEASE PHARMACEUTICAL COMPOSITION MADE OF MICROPARTICLES
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 11020
Estimated Expiration: ⤷ Get Started Free
Patent: 94401
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 09000476
Patent: SLOW RELEASE PHARMACEUTICAL COMPOSITION MADE OF MICROPARTICLES
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 830
Patent: ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ С ПРОЛОНГИРОВАННЫМ ВЫСВОБОЖДЕНИЕМ, ИЗГОТОВЛЕННАЯ ИЗ МИКРОЧАСТИЦ;ФАРМАЦЕВТИЧНА КОМПОЗИЦІЯ З ПРОЛОНГОВАНИМ ВИВІЛЬНЕННЯМ, ВИГОТОВЛЕНА З МІКРОЧАСТИНОК (SLOW RELEASE PHARMACEUTICAL COMPOSITION MADE OF MICROPARTICLES)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TRELSTAR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Germany | 3822459 | Pharmazeutische Zusammensetzung | ⤷ Get Started Free |
| France | 2668707 | PROCEDE DE PREPARATION D'UNE COMPOSITION PHARMACEUTIQUE. | ⤷ Get Started Free |
| Italy | 1225148 | LIBERAZIONE PROLUNGATA E CONTROLLATA DI PEPTIDI INSOLUBILI IN ACQUA | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for TRELSTAR (Triptorelin)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
