FARXIGA Drug Patent Profile
✉ Email this page to a colleague
When do Farxiga patents expire, and when can generic versions of Farxiga launch?
Farxiga is a drug marketed by Astrazeneca Ab and is included in one NDA. There are nineteen patents protecting this drug and one Paragraph IV challenge.
This drug has four hundred and fifty-one patent family members in fifty-two countries.
The generic ingredient in FARXIGA is dapagliflozin. There are twenty-six drug master file entries for this compound. Five suppliers are listed for this compound. Additional details are available on the dapagliflozin profile page.
DrugPatentWatch® Generic Entry Outlook for Farxiga
Farxiga was eligible for patent challenges on January 8, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 16, 2030. This may change due to patent challenges or generic licensing.
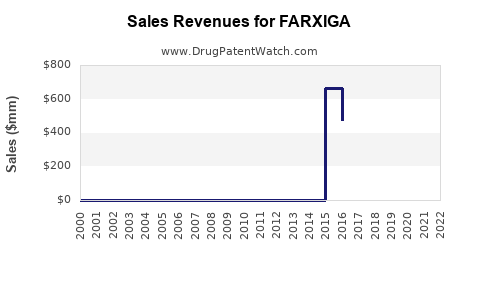
Annual sales in 2022 were $5.3bn, indicating a strong incentive for generic entry.
There have been thirteen patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are sixteen tentative approvals for the generic drug (dapagliflozin), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for FARXIGA?
- What are the global sales for FARXIGA?
- What is Average Wholesale Price for FARXIGA?
Summary for FARXIGA
| International Patents: | 451 |
| US Patents: | 19 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 5 |
| Raw Ingredient (Bulk) Api Vendors: | 2 |
| Clinical Trials: | 50 |
| Patent Applications: | 1 |
| Drug Prices: | Drug price information for FARXIGA |
| Drug Sales Revenues: | Drug sales revenues for FARXIGA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for FARXIGA |
| What excipients (inactive ingredients) are in FARXIGA? | FARXIGA excipients list |
| DailyMed Link: | FARXIGA at DailyMed |
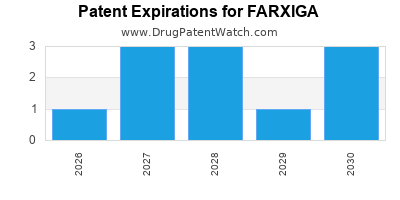
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for FARXIGA
Generic Entry Date for FARXIGA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for FARXIGA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Han Xu, M.D., Ph.D., FAPCR, Sponsor-Investigator, IRB Chair | PHASE2 |
| UnitedHealthcare | PHASE2 |
| The University of Hong Kong | Phase 2 |
Pharmacology for FARXIGA
| Drug Class | Sodium-Glucose Cotransporter 2 Inhibitor |
| Mechanism of Action | Sodium-Glucose Transporter 2 Inhibitors |
Paragraph IV (Patent) Challenges for FARXIGA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| FARXIGA | Tablets | dapagliflozin | 5 mg and 10 mg | 202293 | 20 | 2018-01-08 |
US Patents and Regulatory Information for FARXIGA
FARXIGA is protected by nineteen US patents and four FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of FARXIGA is ⤷ Get Started Free.
This potential generic entry date is based on patent 7,919,598.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-002 | Jan 8, 2014 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-002 | Jan 8, 2014 | RX | Yes | Yes | 8,461,105*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-002 | Jan 8, 2014 | RX | Yes | Yes | 8,685,934*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for FARXIGA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-001 | Jan 8, 2014 | 6,956,026 | ⤷ Get Started Free |
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-001 | Jan 8, 2014 | 7,741,269 | ⤷ Get Started Free |
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-001 | Jan 8, 2014 | 7,563,871 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for FARXIGA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| AstraZeneca AB | Forxiga | dapagliflozin | EMEA/H/C/002322Type 2 diabetes mellitusForxiga is indicated in adults and children aged 10 years and above for the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exerciseas monotherapy when metformin is considered inappropriate due to intolerance.in addition to other medicinal products for the treatment of type 2 diabetes.For study results with respect to combination of therapies, effects on glycaemic control, cardiovascular and renal events, and the populations studied, see sections 4.4, 4.5 and 5.1.Heart failureForxiga is indicated in adults for the treatment of symptomatic chronic heart failure.Chronic kidney diseaseForxiga is indicated in adults for the treatment of chronic kidney disease. | Authorised | no | no | no | 2012-11-11 | |
| AstraZeneca AB | Edistride | dapagliflozin | EMEA/H/C/004161Type 2 diabetes mellitusEdistride is indicated in adults and children aged 10 years and above for the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exerciseas monotherapy when metformin is considered inappropriate due to intolerance.in addition to other medicinal products for the treatment of type 2 diabetes.For study results with respect to combination of therapies, effects on glycaemic control, cardiovascular and renal events, and the populations studied, see sections 4.4, 4.5 and 5.1.Heart failureEdistride is indicated in adults for the treatment of symptomatic chronic heart failure.Chronic kidney diseaseEdistride is indicated in adults for the treatment of chronic kidney disease. | Authorised | no | no | no | 2015-11-09 | |
| Viatris Limited | Dapagliflozin Viatris | dapagliflozin | EMEA/H/C/006006Type 2 diabetes mellitusDapagliflozin Viatris is indicated in adults and children aged 10 years and above for the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise- as monotherapy when metformin is considered inappropriate due to intolerance.- in addition to other medicinal products for the treatment of type 2 diabetes.For study results with respect to combination of therapies, effects on glycaemic control, cardiovascular and renal events, and the populations studied, see sections 4.4, 4.5 and 5.1.Heart failureDapagliflozin Viatris is indicated in adults for the treatment of symptomatic chronic heart failure with reduced ejection fraction.Chronic kidney diseaseDapagliflozin Viatris is indicated in adults for the treatment of chronic kidney disease. | Authorised | yes | no | no | 2023-03-24 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for FARXIGA
When does loss-of-exclusivity occur for FARXIGA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 1730
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 07265246
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0713544
Estimated Expiration: ⤷ Get Started Free
Patent: 2017015106
Estimated Expiration: ⤷ Get Started Free
Patent: 2017021516
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 53344
Estimated Expiration: ⤷ Get Started Free
Patent: 24318
Estimated Expiration: ⤷ Get Started Free
Patent: 85797
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 07001915
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1479287
Estimated Expiration: ⤷ Get Started Free
Patent: 3145773
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 60299
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0141007
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 15738
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 69374
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 8229
Estimated Expiration: ⤷ Get Started Free
Patent: 0428
Estimated Expiration: ⤷ Get Started Free
Patent: 8259
Estimated Expiration: ⤷ Get Started Free
Patent: 5999
Estimated Expiration: ⤷ Get Started Free
Patent: 0900066
Estimated Expiration: ⤷ Get Started Free
Patent: 1171333
Estimated Expiration: ⤷ Get Started Free
Patent: 1490902
Estimated Expiration: ⤷ Get Started Free
Patent: 1791254
Estimated Expiration: ⤷ Get Started Free
Patent: 2091391
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 69374
Estimated Expiration: ⤷ Get Started Free
Patent: 57918
Estimated Expiration: ⤷ Get Started Free
Patent: 45466
Estimated Expiration: ⤷ Get Started Free
Patent: 63807
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 27359
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 5882
Estimated Expiration: ⤷ Get Started Free
Patent: 4180
Estimated Expiration: ⤷ Get Started Free
Patent: 4181
Estimated Expiration: ⤷ Get Started Free
Patent: 4182
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 13889
Estimated Expiration: ⤷ Get Started Free
Patent: 66651
Estimated Expiration: ⤷ Get Started Free
Patent: 37187
Estimated Expiration: ⤷ Get Started Free
Patent: 09545525
Estimated Expiration: ⤷ Get Started Free
Patent: 13209394
Estimated Expiration: ⤷ Get Started Free
Patent: 15071636
Estimated Expiration: ⤷ Get Started Free
Patent: 16172758
Estimated Expiration: ⤷ Get Started Free
Patent: 17222681
Estimated Expiration: ⤷ Get Started Free
Patent: 19059779
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 8566
Estimated Expiration: ⤷ Get Started Free
Patent: 3930
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 9143
Estimated Expiration: ⤷ Get Started Free
Patent: 7155
Estimated Expiration: ⤷ Get Started Free
Patent: 08015377
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 4346
Estimated Expiration: ⤷ Get Started Free
Patent: 9190
Estimated Expiration: ⤷ Get Started Free
Patent: 9195
Estimated Expiration: ⤷ Get Started Free
Patent: 9202
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 6828
Estimated Expiration: ⤷ Get Started Free
Patent: 7770
Estimated Expiration: ⤷ Get Started Free
Patent: 085169
Estimated Expiration: ⤷ Get Started Free
Patent: 221233
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 080349
Estimated Expiration: ⤷ Get Started Free
Patent: 120776
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 012500168
Patent: CRYSTALLINE SOLVATES AND COMPLEXES OF (IS)-1,5-ANHYDRO-L-C-(3-((PHENYL)METHYL)PHENYL)-D-GLUCITOL DERIVATIVES WITH AMINO ACIDS AS SGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 69374
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 69374
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 638
Patent: KRISTALNI SOLVATI DERIVATA (1S)-1,5-ANHIDRO-1-C-(3-((FENIL) METIL) FENIL)-D-GLUCITOLA SA ALKOHOLIMA KAO INHIBITORI SGLT2 ZA TRETMAN DIJABETESA (CRYSTALLINE SOLVATES OF (1S)-1,5-ANHYDRO-1-C-(3-((PHENYL) METHYL) PHENYL)-D-GLUCITOL DERIVATIVES WITH ALCOHOLS AS SGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 2741
Patent: CRYSTALLINE SOLVATES AND COMPLEXES OF (1S) -1, 5-ANHYDRO-1-C- (3- ( (PHENYL) METHYL) PHENYL) -D-GLUCITOL DERIVATIVES WITH AMINO ACIDS AS SGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES
Estimated Expiration: ⤷ Get Started Free
Patent: 201402181S
Patent: CRYSTALLINE SOLVATES AND COMPLEXES OF (1S) -1, 5-ANHYDRO-1-C- (3- ( (PHENYL) METHYL) PHENYL) -D-GLUCITOL DERIVATIVES WITH AMINO ACIDS AS SGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 69374
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0810475
Patent: CRYSTALLINE SOLVATES AND COMPLEXES OF (IS)-1,5-ANHYDRO-L-C-(3-((PHENYL)METHYL)PHENYL)-D-GLUCITOL DERIVATIVES WITH AMINO ACIDS AS SGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1493102
Estimated Expiration: ⤷ Get Started Free
Patent: 090023643
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 21665
Estimated Expiration: ⤷ Get Started Free
Patent: 59862
Estimated Expiration: ⤷ Get Started Free
Patent: 69130
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 21245
Estimated Expiration: ⤷ Get Started Free
Patent: 66876
Estimated Expiration: ⤷ Get Started Free
Patent: 19528
Estimated Expiration: ⤷ Get Started Free
Patent: 0811127
Patent: Crystal structures of SGLT2 inhibitors and processes for preparing same
Estimated Expiration: ⤷ Get Started Free
Patent: 1406743
Patent: Crystal structures of SGLT2 inhibitors and processes for preparing same
Estimated Expiration: ⤷ Get Started Free
Patent: 1509927
Patent: Crystal structures of SGLT2 inhibitors and processes for preparing same
Estimated Expiration: ⤷ Get Started Free
Patent: 1546054
Patent: Crystal structures of SGLT2 inhibitors and processes for preparing same
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 765
Patent: КРИСТАЛЛИЧЕСКИЕ СОЛЬВАТЫ И КОМПЛЕКСЫ ПРОИЗВОДНЫХ (IS)-1,5-АНГИДРО-L-C-(3-((ФЕНИЛ)МЕТИЛ)ФЕНИЛ)-D-ГЛЮЦИТОЛА С АМИНОКИСЛОТАМИ КАК ИНГИБИТОРЫ БЕЛКА SGLT2, ПРИГОДНЫЕ В ЛЕЧЕНИИ ДИАБЕТА;КРИСТАЛІЧНІ СОЛЬВАТИ І КОМПЛЕКСИ ПОХІДНИХ (IS)-1,5-АНГІДРО-L-C-(3-((ФЕНІЛ)МЕТИЛ)ФЕНІЛ)-D-ГЛЮЦИТОЛУ З АМІНОКИСЛОТАМИ ЯК ІНГІБІТОРИ БІЛКА SGLT2, ПРИДАТНІ У ЛІКУВАННІ ДІАБЕТУ (CRYSTALLINE SOLVATES AND COMPLEXES OF (IS) -1, 5-ANHYDRO-L-C- (3- ((PHENYL) METHYL) PHENYL) -D-GLUCITOL DERIVATIVES WITH AMINO ACIDS AS SGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering FARXIGA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| China | 102743340 | ⤷ Get Started Free | |
| South Korea | 101021752 | ⤷ Get Started Free | |
| Australia | 2023202490 | METHODS OF TREATING CHRONIC KIDNEY DISEASE WITH DAPAGLIFLOZIN | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for FARXIGA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2139494 | 132020000000115 | Italy | ⤷ Get Started Free | PRODUCT NAME: SAXAGLIPTIN E DAPAGLIFLOZIN(QTERN); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/16/1108, 20160719 |
| 0996459 | 464 | Finland | ⤷ Get Started Free | |
| 1734971 | CA 2012 00015 | Denmark | ⤷ Get Started Free | PRODUCT NAME: EXENATIDE; REG. NO/DATE: EU/1/11/696/001-002 20110623 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for FARXIGA (Dapagliflozin)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
