Expiring Drug Patents Cheat Sheet
We analyse the patents covering drugs in 134 countries and quickly give you the likely loss-of-exclusivity/generic entry date
European Patent Office: These 33 Drugs Face Patent Expirations and Generic Entry From 2025 - 2026
The content of this page is licensed under a Creative Commons Attribution 4.0 International License.

Generic Entry Dates in Other Countries
Friedman, Yali, "European Patent Office: These 33 Drugs Face Patent Expirations and Generic Entry From 2025 - 2026" DrugPatentWatch.com thinkBiotech, 2025 www.drugpatentwatch.com/p/expiring-drug-patents-generic-entry/.
Media collateral
These estimated drug patent expiration dates and generic entry opportunity dates are calculated from analysis of known patents covering drugs. Many factors can influence early or late generic entry. This information is provided as a rough estimate of generic entry potential and should not be used as an independent source. The methodology is described in this blog post.
When can KOSELUGO (selumetinib sulfate) generic drug versions launch?
Generic name: selumetinib sulfate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: December 21, 2025
Generic Entry Controlled by: European Patent Office Patent 1,968,948
Patent Title: NOUVEAU SEL HYDROGENOSULFATE (NOVEL HYDROGEN SULFATE SALT)
KOSELUGO is a drug marketed by Astrazeneca. There are eight patents protecting this drug.
This drug has two hundred and one patent family members in forty-five countries. There has been litigation on patents covering KOSELUGO
See drug price trends for KOSELUGO.
The generic ingredient in KOSELUGO is selumetinib sulfate. One supplier is listed for this generic product. Additional details are available on the selumetinib sulfate profile page.
When can VYKAT XR (diazoxide choline) generic drug versions launch?
Generic name: diazoxide choline
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: January 05, 2026
Generic Entry Controlled by: European Patent Office Patent 1,968,601
Patent Title: SELS D'OUVREURS DES CANAUX POTASSIQUES ATP-DÉPENDANTS ET LEURS UTILISATIONS (SALTS OF POTASSIUM ATP CHANNEL OPENERS AND USES THEREOF)
VYKAT XR is a drug marketed by Soleno Therap. There are six patents protecting this drug.
This drug has seventy-eight patent family members in twenty-two countries.
The generic ingredient in VYKAT XR is diazoxide choline. There are five drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the diazoxide choline profile page.
When can VYKAT XR (diazoxide choline) generic drug versions launch?
Generic name: diazoxide choline
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: January 05, 2026
Generic Entry Controlled by: European Patent Office Patent 2,404,604
Patent Title: Sels d'ouverture de canal ATP du potassium et leurs utilisations (Salts of potassium ATP channel openers and uses thereof)
VYKAT XR is a drug marketed by Soleno Therap. There are six patents protecting this drug.
This drug has seventy-eight patent family members in twenty-two countries.
The generic ingredient in VYKAT XR is diazoxide choline. There are five drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the diazoxide choline profile page.
When can VYKAT XR (diazoxide choline) generic drug versions launch?
Generic name: diazoxide choline
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: January 05, 2026
Generic Entry Controlled by: European Patent Office Patent 3,545,958
Patent Title: SELS D'OUVERTURE DE CANAL ATP DU POTASSIUM ET LEURS UTILISATIONS (SALTS OF POTASSIUM ATP CHANNEL OPENERS AND USES THEREOF)
VYKAT XR is a drug marketed by Soleno Therap. There are six patents protecting this drug.
This drug has seventy-eight patent family members in twenty-two countries.
The generic ingredient in VYKAT XR is diazoxide choline. There are five drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the diazoxide choline profile page.
When can CAMCEVI KIT (leuprolide mesylate) generic drug versions launch?
Generic name: leuprolide mesylate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: January 18, 2026
Generic Entry Controlled by: European Patent Office Patent 1,984,009
Patent Title: COMPOSITIONS PHARMACEUTIQUES A STABILITE AMELIOREE (PHARMACEUTICAL COMPOSITIONS WITH ENHANCED STABILITY)
CAMCEVI KIT is a drug marketed by Accord. There are five patents protecting this drug.
This drug has thirty-nine patent family members in nineteen countries.
The generic ingredient in CAMCEVI KIT is leuprolide mesylate. There are twenty-two drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the leuprolide mesylate profile page.
When can RAPIVAB (peramivir) generic drug versions launch?
Generic name: peramivir
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: February 13, 2026
Generic Entry Controlled by: European Patent Office Patent 1,986,626
Patent Title: TRAITEMENTS ANTIVIRAUX INTRAVEINEUX (INTRAVENOUS ANTIVIRAL TREATMENTS)
RAPIVAB is a drug marketed by Biocryst. There are two patents protecting this drug.
This drug has forty-three patent family members in fourteen countries.
See drug price trends for RAPIVAB.
The generic ingredient in RAPIVAB is peramivir. There is one drug master file entry for this API. One supplier is listed for this generic product. Additional details are available on the peramivir profile page.
When can TUZISTRA XR (chlorpheniramine polistirex; codeine polistirex) generic drug versions launch?
Generic name: chlorpheniramine polistirex; codeine polistirex
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: March 16, 2026
Generic Entry Controlled by: European Patent Office Patent 2,428,205
Patent Title: Formulations à libération modifiée contenant des complexes médicament - résine échangeuse d'ions (Modified release formulations containing drug-ion exchange resin complexes)
TUZISTRA XR is a drug marketed by Tris Pharma Inc. There are two patents protecting this drug.
This drug has twenty-one patent family members in fourteen countries. There has been litigation on patents covering TUZISTRA XR
See drug price trends for TUZISTRA XR.
The generic ingredient in TUZISTRA XR is chlorpheniramine polistirex; codeine polistirex. There are twenty-nine drug master file entries for this API. Additional details are available on the chlorpheniramine polistirex; codeine polistirex profile page.
When can AMYVID (florbetapir f-18) generic drug versions launch?
Generic name: florbetapir f-18
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: March 30, 2026
Generic Entry Controlled by: European Patent Office Patent 1,999,109
AMYVID is a drug marketed by Avid Radiopharms Inc. There are two patents protecting this drug.
This drug has fifty-one patent family members in thirty-three countries. There has been litigation on patents covering AMYVID
The generic ingredient in AMYVID is florbetapir f-18. One supplier is listed for this generic product. Additional details are available on the florbetapir f-18 profile page.
When can LASTACAFT (alcaftadine) generic drug versions launch?
Generic name: alcaftadine
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: March 31, 2026
Generic Entry Controlled by: European Patent Office Patent 2,004,196
Patent Title: TRAITEMENTS DE L'ALLERGIE OCULAIRE (OCULAR ALLERGY TREATMENTS)
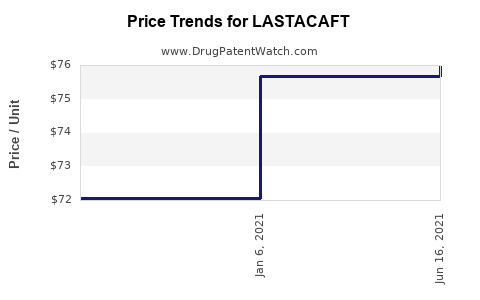
This drug has forty-six patent family members in thirty countries. There has been litigation on patents covering LASTACAFT
See drug price trends for LASTACAFT.
The generic ingredient in LASTACAFT is alcaftadine. There are six drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the alcaftadine profile page.
When can LASTACAFT (alcaftadine) generic drug versions launch?
Generic name: alcaftadine
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: March 31, 2026
Generic Entry Controlled by: European Patent Office Patent 3,150,209
Patent Title: TRAITEMENTS D'ALLERGIE OCULAIRE (OCULAR ALLERGY TREATMENTS)

This drug has forty-six patent family members in thirty countries. There has been litigation on patents covering LASTACAFT
See drug price trends for LASTACAFT.
The generic ingredient in LASTACAFT is alcaftadine. There are six drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the alcaftadine profile page.
When can TYKERB (lapatinib ditosylate) generic drug versions launch?
Generic name: lapatinib ditosylate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: April 18, 2026
Generic Entry Controlled by: European Patent Office Patent 1,871,347
Patent Title: PREPARATION PHARMACEUTIQUE (PHARMACEUTICAL COMPOSITION)
TYKERB is a drug marketed by Novartis. There is one patent protecting this drug and one Paragraph IV challenge.
This drug has twenty-eight patent family members in twenty-six countries.
See drug price trends for TYKERB.
The generic ingredient in TYKERB is lapatinib ditosylate. There are seven drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the lapatinib ditosylate profile page.
When can APTIOM (eslicarbazepine acetate) generic drug versions launch?
Generic name: eslicarbazepine acetate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: April 21, 2026
Generic Entry Controlled by: European Patent Office Patent 2,319,836
Patent Title: Reduction catalytique asymetrique d'oxcarbazepine (Asymmetric catalytic reduction of oxcarbazepine)
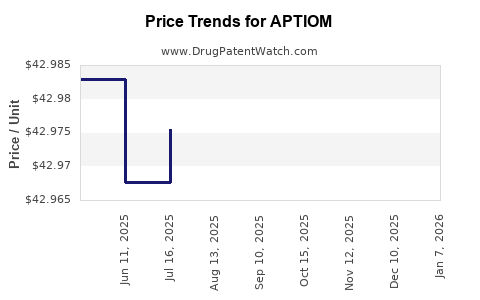
This drug has one hundred patent family members in twenty-six countries. There has been litigation on patents covering APTIOM
See drug price trends for APTIOM.
The generic ingredient in APTIOM is eslicarbazepine acetate. There are twelve drug master file entries for this API. Eight suppliers are listed for this generic product. Additional details are available on the eslicarbazepine acetate profile page.
When can ORENITRAM (treprostinil diolamine) generic drug versions launch?
Generic name: treprostinil diolamine
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: April 27, 2026
Generic Entry Controlled by: European Patent Office Patent 2,010,189
Patent Title: DISPOSITIF OSMOTIQUE D'ADMINISTRATION D'UN MÉDICAMENT COMPRENANT UN PROMOTEUR DE LA LIBÉRATION (OSMOTIC DRUG DELIVERY SYSTEM COMPRISING RELEASE ENHANCING AGENT)
ORENITRAM is a drug marketed by United Therap. There are nine patents protecting this drug and two Paragraph IV challenges.
This drug has fifty-nine patent family members in eight countries. There has been litigation on patents covering ORENITRAM
See drug price trends for ORENITRAM.
The generic ingredient in ORENITRAM is treprostinil diolamine. There are nineteen drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the treprostinil diolamine profile page.
When can TRADJENTA (linagliptin) generic drug versions launch?
Generic name: linagliptin
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: May 04, 2026
Generic Entry Controlled by: European Patent Office Patent 1,852,108
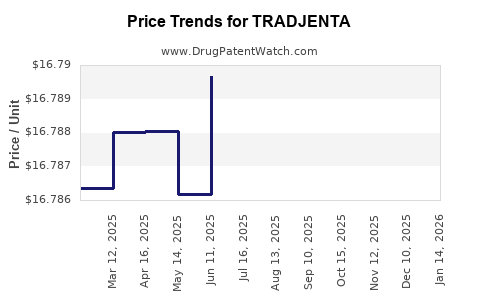
This drug has four hundred and eighty-six patent family members in forty-five countries. There has been litigation on patents covering TRADJENTA
See drug price trends for TRADJENTA.
The generic ingredient in TRADJENTA is linagliptin. There are nineteen drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the linagliptin profile page.
When can TRADJENTA (linagliptin) generic drug versions launch?
Generic name: linagliptin
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: May 04, 2026
Generic Entry Controlled by: European Patent Office Patent 2,023,902

This drug has four hundred and eighty-six patent family members in forty-five countries. There has been litigation on patents covering TRADJENTA
See drug price trends for TRADJENTA.
The generic ingredient in TRADJENTA is linagliptin. There are nineteen drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the linagliptin profile page.
When can TRADJENTA (linagliptin) generic drug versions launch?
Generic name: linagliptin
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: May 04, 2026
Generic Entry Controlled by: European Patent Office Patent 2,277,509

This drug has four hundred and eighty-six patent family members in forty-five countries. There has been litigation on patents covering TRADJENTA
See drug price trends for TRADJENTA.
The generic ingredient in TRADJENTA is linagliptin. There are nineteen drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the linagliptin profile page.
When can TRADJENTA (linagliptin) generic drug versions launch?
Generic name: linagliptin
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: May 04, 2026
Generic Entry Controlled by: European Patent Office Patent 2,283,819

This drug has four hundred and eighty-six patent family members in forty-five countries. There has been litigation on patents covering TRADJENTA
See drug price trends for TRADJENTA.
The generic ingredient in TRADJENTA is linagliptin. There are nineteen drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the linagliptin profile page.
When can TRINTELLIX (vortioxetine hydrobromide) generic drug versions launch?
Generic name: vortioxetine hydrobromide
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: June 16, 2026
Generic Entry Controlled by: European Patent Office Patent 2,044,043
Patent Title: 1-[2-(2, 4-DIMETHYLPHENYLSULFANYL)-PHENYL]PIPERAZINE COMME COMPOSE PRESENTANT UNE ACTIVITE SUR LA SEROTONINE, 5-HT3 ET 5-HT1A POUR LE TRAITEMENT DU DEFICIT COGNITIF (1- Ý[- (2, 4-DIMETHYLPHENYLSULFANYL) -PHENYL]PIPERAZINE AS A COMPOUND WITH COMBINED SEROTONIN REUPTAKE, 5-HT3 AND 5-HT1A ACTIVITY FOR THE TREATMENT OF COGNITIVE IMPAIRMENT)
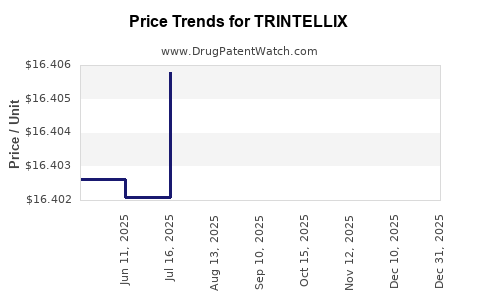
This drug has two hundred and seventeen patent family members in forty-two countries. There has been litigation on patents covering TRINTELLIX
See drug price trends for TRINTELLIX.
The generic ingredient in TRINTELLIX is vortioxetine hydrobromide. There are sixteen drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the vortioxetine hydrobromide profile page.
When can EMBEDA (morphine sulfate; naltrexone hydrochloride) generic drug versions launch?
Generic name: morphine sulfate; naltrexone hydrochloride
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: June 19, 2026
Generic Entry Controlled by: European Patent Office Patent 2,484,346
EMBEDA is a drug marketed by Alpharma Pharms. There are nine patents protecting this drug and four Paragraph IV challenges.
This drug has seventy-four patent family members in twenty-three countries.
See drug price trends for EMBEDA.
The generic ingredient in EMBEDA is morphine sulfate; naltrexone hydrochloride. There are twenty-three drug master file entries for this API. Additional details are available on the morphine sulfate; naltrexone hydrochloride profile page.
When can EMBEDA (morphine sulfate; naltrexone hydrochloride) generic drug versions launch?
Generic name: morphine sulfate; naltrexone hydrochloride
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: June 19, 2026
Generic Entry Controlled by: European Patent Office Patent 2,526,932
EMBEDA is a drug marketed by Alpharma Pharms. There are nine patents protecting this drug and four Paragraph IV challenges.
This drug has seventy-four patent family members in twenty-three countries.
See drug price trends for EMBEDA.
The generic ingredient in EMBEDA is morphine sulfate; naltrexone hydrochloride. There are twenty-three drug master file entries for this API. Additional details are available on the morphine sulfate; naltrexone hydrochloride profile page.
When can TEKTURNA HCT (aliskiren hemifumarate; hydrochlorothiazide) generic drug versions launch?
Generic name: aliskiren hemifumarate; hydrochlorothiazide
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: June 23, 2026
Generic Entry Controlled by: European Patent Office Patent 3,391,878

This drug has thirty-two patent family members in twenty-five countries. There has been litigation on patents covering TEKTURNA HCT
See drug price trends for TEKTURNA HCT.
The generic ingredient in TEKTURNA HCT is aliskiren hemifumarate; hydrochlorothiazide. There are four drug master file entries for this API. Additional details are available on the aliskiren hemifumarate; hydrochlorothiazide profile page.
When can VAFSEO (vadadustat) generic drug versions launch?
Generic name: vadadustat
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: June 26, 2026
Generic Entry Controlled by: European Patent Office Patent 3,323,807
Patent Title: INHIBITEURS DE PROLYL HYDROXYLASE ET PROCÉDÉS D'UTILISATION (PROLYL HYDROXYLASE INHIBITORS AND METHODS OF USE)
VAFSEO is a drug marketed by Akebia. There are thirteen patents protecting this drug.
This drug has two hundred and fifty-eight patent family members in forty-eight countries. There has been litigation on patents covering VAFSEO
The generic ingredient in VAFSEO is vadadustat. One supplier is listed for this generic product. Additional details are available on the vadadustat profile page.
When can EXFORGE HCT (amlodipine besylate; hydrochlorothiazide; valsartan) generic drug versions launch?
Generic name: amlodipine besylate; hydrochlorothiazide; valsartan
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: June 27, 2026
Generic Entry Controlled by: European Patent Office Patent 2,037,893

This drug has two hundred and fifty-eight patent family members in forty-eight countries. There has been litigation on patents covering EXFORGE HCT
See drug price trends for EXFORGE HCT.
The generic ingredient in EXFORGE HCT is amlodipine besylate; hydrochlorothiazide; valsartan. There are fifty drug master file entries for this API. Five suppliers are listed for this generic product. Additional details are available on the amlodipine besylate; hydrochlorothiazide; valsartan profile page.
When can BYDUREON (exenatide synthetic) generic drug versions launch?
Generic name: exenatide synthetic
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: June 28, 2026
Generic Entry Controlled by: European Patent Office Patent 2,069,374

This drug has three hundred and forty-six patent family members in forty-eight countries. There has been litigation on patents covering BYDUREON
See drug price trends for BYDUREON.
The generic ingredient in BYDUREON is exenatide synthetic. There are seven drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the exenatide synthetic profile page.
When can EPIDUO (adapalene; benzoyl peroxide) generic drug versions launch?
Generic name: adapalene; benzoyl peroxide
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: July 13, 2026
Generic Entry Controlled by: European Patent Office Patent 2,046,318
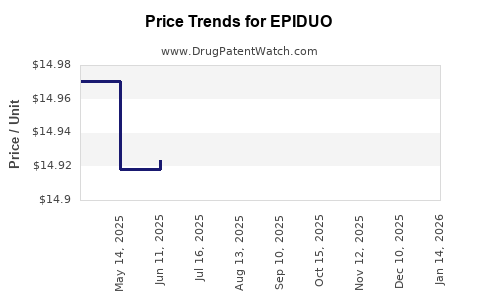
This drug has sixty-eight patent family members in twenty-five countries. There has been litigation on patents covering EPIDUO
See drug price trends for EPIDUO.
The generic ingredient in EPIDUO is adapalene; benzoyl peroxide. There are twelve drug master file entries for this API. Thirteen suppliers are listed for this generic product. Additional details are available on the adapalene; benzoyl peroxide profile page.
When can EPIDUO FORTE (adapalene; benzoyl peroxide) generic drug versions launch?
Generic name: adapalene; benzoyl peroxide
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: July 13, 2026
Generic Entry Controlled by: European Patent Office Patent 2,046,318
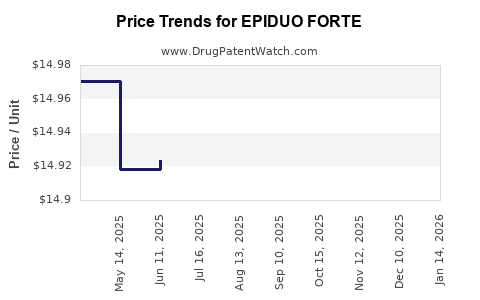
This drug has sixty-eight patent family members in twenty-five countries. There has been litigation on patents covering EPIDUO FORTE
See drug price trends for EPIDUO FORTE.
The generic ingredient in EPIDUO FORTE is adapalene; benzoyl peroxide. There are twelve drug master file entries for this API. Thirteen suppliers are listed for this generic product. Additional details are available on the adapalene; benzoyl peroxide profile page.
When can RASUVO (methotrexate) generic drug versions launch?
Generic name: methotrexate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: July 21, 2026
Generic Entry Controlled by: European Patent Office Patent 2,046,332
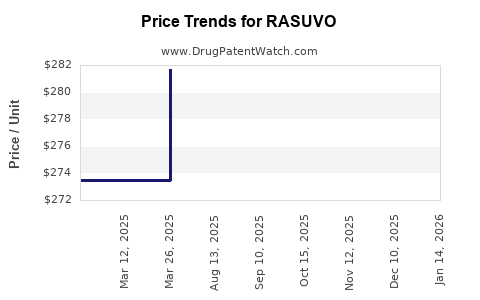
This drug has twenty-nine patent family members in twenty-one countries. There has been litigation on patents covering RASUVO
See drug price trends for RASUVO.
The generic ingredient in RASUVO is methotrexate. There are twenty drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the methotrexate profile page.
When can OLYSIO (simeprevir sodium) generic drug versions launch?
Generic name: simeprevir sodium
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: July 28, 2026
Generic Entry Controlled by: European Patent Office Patent 2,322,516
Patent Title: Composés intermédiaires pour la préparation d'inhibiteurs macrocycliques du virus de l'hépatite C (Intermediates for the preparation of Macrocyclic inhibitors of hepatitis c virus)
OLYSIO is a drug marketed by Janssen Prods. There are nine patents protecting this drug.
This drug has one hundred and forty patent family members in forty-three countries.
See drug price trends for OLYSIO.
The generic ingredient in OLYSIO is simeprevir sodium. There is one drug master file entry for this API. Additional details are available on the simeprevir sodium profile page.
When can OLYSIO (simeprevir sodium) generic drug versions launch?
Generic name: simeprevir sodium
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: July 28, 2026
Generic Entry Controlled by: European Patent Office Patent 2,937,339
Patent Title: Inhibiteurs macrocycliques du virus de l'hépatite C (Macrocylic inhibitors of hepatitis c virus)
OLYSIO is a drug marketed by Janssen Prods. There are nine patents protecting this drug.
This drug has one hundred and forty patent family members in forty-three countries.
See drug price trends for OLYSIO.
The generic ingredient in OLYSIO is simeprevir sodium. There is one drug master file entry for this API. Additional details are available on the simeprevir sodium profile page.
When can EPSOLAY (benzoyl peroxide) generic drug versions launch?
Generic name: benzoyl peroxide
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: August 02, 2026
Generic Entry Controlled by: European Patent Office Patent 1,919,606
Patent Title: REVETEMENT PAR UN OXYDE METALLIQUE D'INGREDIENTS HYDROINSOLUBLES (METAL OXIDE COATING OF WATER INSOLUBLE INGREDIENTS)
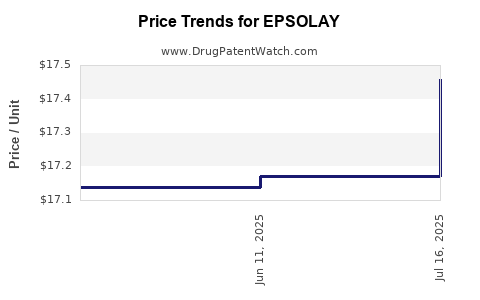
This drug has fifty-one patent family members in fifteen countries. There has been litigation on patents covering EPSOLAY
See drug price trends for EPSOLAY.
The generic ingredient in EPSOLAY is benzoyl peroxide. There are seventeen drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the benzoyl peroxide profile page.
When can EPSOLAY (benzoyl peroxide) generic drug versions launch?
Generic name: benzoyl peroxide
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: August 02, 2026
Generic Entry Controlled by: European Patent Office Patent 2,431,088
Patent Title: Revêtement d'oxyde métallique d'ingrédients insolubles dans l'eau (Metal oxide coating of water insoluble ingredients)

This drug has fifty-one patent family members in fifteen countries. There has been litigation on patents covering EPSOLAY
See drug price trends for EPSOLAY.
The generic ingredient in EPSOLAY is benzoyl peroxide. There are seventeen drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the benzoyl peroxide profile page.
When can EPSOLAY (benzoyl peroxide) generic drug versions launch?
Generic name: benzoyl peroxide
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: August 02, 2026
Generic Entry Controlled by: European Patent Office Patent 2,431,089
Patent Title: Revêtement d'oxyde métallique d'ingrédients insolubles dans l'eau (Metal oxide coating of water insoluble ingredients)

This drug has fifty-one patent family members in fifteen countries. There has been litigation on patents covering EPSOLAY
See drug price trends for EPSOLAY.
The generic ingredient in EPSOLAY is benzoyl peroxide. There are seventeen drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the benzoyl peroxide profile page.
When can BRILINTA (ticagrelor) generic drug versions launch?
Generic name: ticagrelor
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: August 21, 2026
Generic Entry Controlled by: European Patent Office Patent 2,056,832
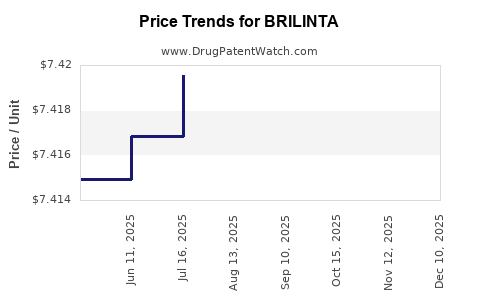
This drug has one hundred and forty-seven patent family members in forty-four countries. There has been litigation on patents covering BRILINTA
See drug price trends for BRILINTA.
The generic ingredient in BRILINTA is ticagrelor. There are twenty-one drug master file entries for this API. Twenty-five suppliers are listed for this generic product. Additional details are available on the ticagrelor profile page.
When can ESBRIET (pirfenidone) generic drug versions launch?
Generic name: pirfenidone
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: September 22, 2026
Generic Entry Controlled by: European Patent Office Patent 1,940,364
ESBRIET is a drug marketed by Genentech Inc. There are twenty patents protecting this drug and two Paragraph IV challenges. One tentatively approved generic is ready to enter the market.
This drug has two hundred and sixty-six patent family members in forty-six countries. There has been litigation on patents covering ESBRIET
See drug price trends for ESBRIET.
The generic ingredient in ESBRIET is pirfenidone. There are twenty-three drug master file entries for this API. Twenty-four suppliers are listed for this generic product. Additional details are available on the pirfenidone profile page.
When can ESBRIET (pirfenidone) generic drug versions launch?
Generic name: pirfenidone
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: September 22, 2026
Generic Entry Controlled by: European Patent Office Patent 2,431,025
ESBRIET is a drug marketed by Genentech Inc. There are twenty patents protecting this drug and two Paragraph IV challenges. One tentatively approved generic is ready to enter the market.
This drug has two hundred and sixty-six patent family members in forty-six countries. There has been litigation on patents covering ESBRIET
See drug price trends for ESBRIET.
The generic ingredient in ESBRIET is pirfenidone. There are twenty-three drug master file entries for this API. Twenty-four suppliers are listed for this generic product. Additional details are available on the pirfenidone profile page.
When can ZUNVEYL (benzgalantamine gluconate) generic drug versions launch?
Generic name: benzgalantamine gluconate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: September 22, 2026
Generic Entry Controlled by: European Patent Office Patent 1,940,817
Patent Title: AMPLIFICATEURS CHOLINERGIQUES DE PERMÉABILITÉ DE LA BARRIÈRE SANG-CERVEAU AMÉLIORÉE POUR LE TRAITEMENT DE MALADIES ACCOMPAGNÉES D'UNE DÉFICIENCE COGNITIVE (CHOLINERGIC ENHANCERS WITH IMPROVED BLOOD-BRAIN BARRIER PERMEABILITY FOR THE TREATMENT OF DISEASES ACCOMPANIED BY COGNITIVE IMPAIRMENT)
ZUNVEYL is a drug marketed by Alpha Cognition. There are three patents protecting this drug.
This drug has twenty-six patent family members in seventeen countries. There has been litigation on patents covering ZUNVEYL
The generic ingredient in ZUNVEYL is benzgalantamine gluconate. One supplier is listed for this generic product. Additional details are available on the benzgalantamine gluconate profile page.
When can CRESEMBA (isavuconazonium sulfate) generic drug versions launch?
Generic name: isavuconazonium sulfate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: September 25, 2026
Generic Entry Controlled by: European Patent Office Patent 1,902,708
Patent Title: Compositions pharmaceutiques stabilisées et solides contenant au moins un médicament et procédé d'élaboration (Drug comprising stabilized pharmaceutical solid compositions and processes for their preparation)
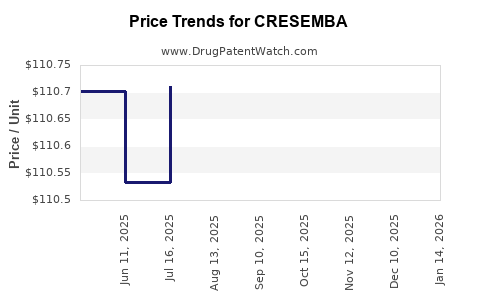
This drug has thirty-two patent family members in nineteen countries. There has been litigation on patents covering CRESEMBA
See drug price trends for CRESEMBA.
The generic ingredient in CRESEMBA is isavuconazonium sulfate. One supplier is listed for this generic product. Additional details are available on the isavuconazonium sulfate profile page.
When can LYNPARZA (olaparib) generic drug versions launch?
Generic name: olaparib
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: October 17, 2026
Generic Entry Controlled by: European Patent Office Patent 2,064,189
LYNPARZA is a drug marketed by Astrazeneca. There are twelve patents protecting this drug. Three tentatively approved generics are ready to enter the market.
This drug has two hundred and fifty-four patent family members in fifty-two countries. There has been litigation on patents covering LYNPARZA
See drug price trends for LYNPARZA.
The generic ingredient in LYNPARZA is olaparib. There are three drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the olaparib profile page.
When can LYNPARZA (olaparib) generic drug versions launch?
Generic name: olaparib
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: October 17, 2026
Generic Entry Controlled by: European Patent Office Patent 2,374,800
LYNPARZA is a drug marketed by Astrazeneca. There are twelve patents protecting this drug. Three tentatively approved generics are ready to enter the market.
This drug has two hundred and fifty-four patent family members in fifty-two countries. There has been litigation on patents covering LYNPARZA
See drug price trends for LYNPARZA.
The generic ingredient in LYNPARZA is olaparib. There are three drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the olaparib profile page.
When can AXUMIN (fluciclovine f-18) generic drug versions launch?
Generic name: fluciclovine f-18
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: November 28, 2026
Generic Entry Controlled by: European Patent Office Patent 1,978,015
Patent Title: COMPOSE PRECURSEUR DE COMPOSE ORGANIQUE MARQUE A L'HALOGENE RADIOACTIF (PRECURSOR COMPOUND OF RADIOACTIVE HALOGEN LABELED ORGANIC COMPOUND)
AXUMIN is a drug marketed by Blue Earth. There are eight patents protecting this drug.
This drug has thirty patent family members in sixteen countries. There has been litigation on patents covering AXUMIN
The generic ingredient in AXUMIN is fluciclovine f-18. One supplier is listed for this generic product. Additional details are available on the fluciclovine f-18 profile page.
When can ZTALMY (ganaxolone) generic drug versions launch?
Generic name: ganaxolone
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: November 28, 2026
Generic Entry Controlled by: European Patent Office Patent 1,959,966
Patent Title: FORMES GALÉNIQUES DE GANAXOLONE ET PROCÉDÉS DE PREPARATION ET D'UTILISATION DE CELLES-CI (GANAXOLONE FORMULATIONS AND METHODS FOR THE MAKING AND USE THEREOF)
ZTALMY is a drug marketed by Marinus. There are eleven patents protecting this drug.
This drug has forty-eight patent family members in sixteen countries. There has been litigation on patents covering ZTALMY
See drug price trends for ZTALMY.
The generic ingredient in ZTALMY is ganaxolone. One supplier is listed for this generic product. Additional details are available on the ganaxolone profile page.
When can SIRTURO (bedaquiline fumarate) generic drug versions launch?
Generic name: bedaquiline fumarate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: December 05, 2026
Generic Entry Controlled by: European Patent Office Patent 2,086,940
SIRTURO is a drug marketed by Janssen Therap. There are two patents protecting this drug.
This drug has ninety-seven patent family members in thirty-nine countries.
See drug price trends for SIRTURO.
The generic ingredient in SIRTURO is bedaquiline fumarate. There is one drug master file entry for this API. One supplier is listed for this generic product. Additional details are available on the bedaquiline fumarate profile page.
When can XERMELO (telotristat etiprate) generic drug versions launch?
Generic name: telotristat etiprate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: December 12, 2026
Generic Entry Controlled by: European Patent Office Patent 2,589,600
XERMELO is a drug marketed by Tersera. There are five patents protecting this drug.
This drug has seventy patent family members in twenty-nine countries.
See drug price trends for XERMELO.
The generic ingredient in XERMELO is telotristat etiprate. One supplier is listed for this generic product. Additional details are available on the telotristat etiprate profile page.
When can PICATO (ingenol mebutate) generic drug versions launch?
Generic name: ingenol mebutate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: December 18, 2026
Generic Entry Controlled by: European Patent Office Patent 2,399,571
Patent Title: Compositions thérapeutiques comprenant de l'ingénol-2-angelate (Therapeutic compositions comprising ingenol-3-angelate)

This drug has thirty-five patent family members in twenty-one countries. There has been litigation on patents covering PICATO
See drug price trends for PICATO.
The generic ingredient in PICATO is ingenol mebutate. There are three drug master file entries for this API. Additional details are available on the ingenol mebutate profile page.
When can AMELUZ (aminolevulinic acid hydrochloride) generic drug versions launch?
Generic name: aminolevulinic acid hydrochloride
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: December 22, 2026
Generic Entry Controlled by: European Patent Office Patent 1,938,801
AMELUZ is a drug marketed by Biofrontera. There are three patents protecting this drug.
This drug has twenty-nine patent family members in eighteen countries.
See drug price trends for AMELUZ.
The generic ingredient in AMELUZ is aminolevulinic acid hydrochloride. There are six drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the aminolevulinic acid hydrochloride profile page.
European Patent Office Branded and Generic Drug Markets: Assessment, Regulatory Opportunities, and Challenges
More… ↓
DrugPatentWatch cited by CNN, NEJM, Nature Journals, and more …

Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
