RAPIVAB Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Rapivab, and what generic alternatives are available?
Rapivab is a drug marketed by Biocryst and is included in one NDA. There are two patents protecting this drug.
This drug has forty-three patent family members in fourteen countries.
The generic ingredient in RAPIVAB is peramivir. There is one drug master file entry for this compound. One supplier is listed for this compound. Additional details are available on the peramivir profile page.
DrugPatentWatch® Generic Entry Outlook for Rapivab
Rapivab was eligible for patent challenges on December 19, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be February 12, 2027. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for RAPIVAB?
- What are the global sales for RAPIVAB?
- What is Average Wholesale Price for RAPIVAB?
Summary for RAPIVAB
| International Patents: | 43 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 79 |
| Clinical Trials: | 3 |
| Patent Applications: | 1,531 |
| Drug Prices: | Drug price information for RAPIVAB |
| What excipients (inactive ingredients) are in RAPIVAB? | RAPIVAB excipients list |
| DailyMed Link: | RAPIVAB at DailyMed |
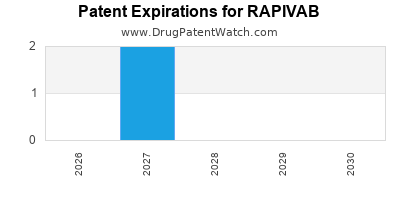

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for RAPIVAB
Generic Entry Date for RAPIVAB*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for RAPIVAB
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Oxford | Phase 2 |
| BioCryst Pharmaceuticals | Phase 3 |
| Johns Hopkins University | Phase 4 |
US Patents and Regulatory Information for RAPIVAB
RAPIVAB is protected by five US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of RAPIVAB is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biocryst | RAPIVAB | peramivir | SOLUTION;INTRAVENOUS | 206426-001 | Dec 19, 2014 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Biocryst | RAPIVAB | peramivir | SOLUTION;INTRAVENOUS | 206426-001 | Dec 19, 2014 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for RAPIVAB
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Biocryst | RAPIVAB | peramivir | SOLUTION;INTRAVENOUS | 206426-001 | Dec 19, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| Biocryst | RAPIVAB | peramivir | SOLUTION;INTRAVENOUS | 206426-001 | Dec 19, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for RAPIVAB
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Biocryst | Alpivab | peramivir | EMEA/H/C/004299Alpivab is indicated for the treatment of uncomplicated influenza in adults and children from the age of 2 years. | Withdrawn | no | no | no | 2018-04-13 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for RAPIVAB
When does loss-of-exclusivity occur for RAPIVAB?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 06341592
Patent: Intramuscular antiviral treatments
Estimated Expiration: ⤷ Get Started Free
Patent: 07215156
Patent: Intravenous antiviral treatments
Estimated Expiration: ⤷ Get Started Free
Patent: 13216632
Patent: INTRAVENOUS ANTIVIRAL TREATMENTS
Estimated Expiration: ⤷ Get Started Free
Patent: 16262644
Patent: INTRAVENOUS ANTIVIRAL TREATMENTS
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0621552
Patent: tratamentos antivirais intramusculares
Estimated Expiration: ⤷ Get Started Free
Patent: 0707769
Patent: tratamentos antivirais intravenosos
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 42260
Patent: TRAITEMENTS ANTIVIRAUX INTRAVEINEUX (INTRAVENOUS ANTIVIRAL TREATMENTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 49090
Patent: TRAITEMENTS ANTIVIRAUX INTRAMUSCULAIRES (INTRAMUSCULAR ANTIVIRAL TREATMENTS)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1420948
Patent: Intramuscular antiviral treatments
Estimated Expiration: ⤷ Get Started Free
Patent: 4784166
Patent: Antiviral compound, dosage form including same, and uses thereof
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 5483
Patent: ВНУТРИВЕННЫЙ СПОСОБ ЛЕЧЕНИЯ СЕЗОННОГО ГРИППА (INTRAVENOUS METHOD FOR TREATING A SEASONAL INFLUENZA)
Estimated Expiration: ⤷ Get Started Free
Patent: 0870263
Patent: ВНУТРИВЕННЫЙ СПОСОБ ЛЕЧЕНИЯ ВИРУСНОЙ ИНФЕКЦИИ
Estimated Expiration: ⤷ Get Started Free
Patent: 0870430
Patent: ВНУТРИМЫШЕЧНЫЕ АНТИВИРУСНЫЕ СПОСОБЫ ЛЕЧЕНИЯ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 86626
Patent: TRAITEMENTS ANTIVIRAUX INTRAVEINEUX (INTRAVENOUS ANTIVIRAL TREATMENTS)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 12250
Patent: 抗病毒化合物、包含該化合物的劑型及其用途 (ANTIVIRAL COMPOUNDS, UNIT DOSAGE FORM COMPRISING SAID COMPOUNDS AND USE THEREOF)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 73202
Estimated Expiration: ⤷ Get Started Free
Patent: 09533428
Estimated Expiration: ⤷ Get Started Free
Patent: 09538822
Estimated Expiration: ⤷ Get Started Free
Patent: 13256527
Patent: INTRAVENOUS ANTIVIRAL TREATMENT
Estimated Expiration: ⤷ Get Started Free
Patent: 15180695
Patent: 静脈におけるウイルスの治療 (INTRAVENOUS ANTIVIRAL TREATMENT)
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 6063
Patent: INTRAVENOUS ANTIVIRAL TREATMENTS
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 3640
Patent: TRATAMIENTOS ANTIVIRALES INTRAVENOSOS. (INTRAVENOUS ANTIVIRAL TREATMENTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 08010394
Patent: TRATAMIENTOS ANTIVIRALES INTRAVENOSOS. (INTRAVENOUS ANTIVIRAL TREATMENTS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 08013140
Patent: TRATAMIENTOS ANTIVIRALES INTRAMUSCULARES. (INTRAMUSCULAR ANTIVIRAL TREATMENTS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 20002008
Patent: TRATAMIENTOS ANTIVIRALES INTRAVENOSOS. (INTRAVENOUS ANTIVIRAL TREATMENTS.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 0538
Patent: Intravenous antiviral treatments
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0809012
Patent: Intravenous antiviral treatments
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1992585
Estimated Expiration: ⤷ Get Started Free
Patent: 2194015
Estimated Expiration: ⤷ Get Started Free
Patent: 2267754
Estimated Expiration: ⤷ Get Started Free
Patent: 2323339
Estimated Expiration: ⤷ Get Started Free
Patent: 2475176
Estimated Expiration: ⤷ Get Started Free
Patent: 080096829
Patent: INTRAVENOUS ANTIVIRAL TREATMENTS
Estimated Expiration: ⤷ Get Started Free
Patent: 140132778
Patent: INTRAVENOUS ANTIVIRAL TREATMENTS
Estimated Expiration: ⤷ Get Started Free
Patent: 160129105
Patent: 정맥내 항바이러스 치료 (INTRAVENOUS ANTIVIRAL TREATMENTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 180024027
Patent: 정맥내 항바이러스 치료 (INTRAVENOUS ANTIVIRAL TREATMENTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 190072681
Patent: 정맥내 항바이러스 치료 (INTRAVENOUS ANTIVIRAL TREATMENTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 200143519
Patent: 정맥내 항바이러스 치료 (INTRAVENOUS ANTIVIRAL TREATMENTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 210076189
Patent: 정맥내 항바이러스 치료 (INTRAVENOUS ANTIVIRAL TREATMENTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 210135632
Patent: 정맥내 항바이러스 치료 (INTRAVENOUS ANTIVIRAL TREATMENTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 230003248
Patent: 정맥내 항바이러스 치료 (INTRAVENOUS ANTIVIRAL TREATMENTS)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering RAPIVAB around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2013256527 | INTRAVENOUS ANTIVIRAL TREATMENT | ⤷ Get Started Free |
| Japan | 2009538822 | ⤷ Get Started Free | |
| New Zealand | 570538 | Intravenous antiviral treatments | ⤷ Get Started Free |
| Japan | 2009533428 | ⤷ Get Started Free | |
| Mexico | 2008010394 | TRATAMIENTOS ANTIVIRALES INTRAVENOSOS. (INTRAVENOUS ANTIVIRAL TREATMENTS.) | ⤷ Get Started Free |
| Hong Kong | 1212250 | 抗病毒化合物、包含該化合物的劑型及其用途 (ANTIVIRAL COMPOUNDS, UNIT DOSAGE FORM COMPRISING SAID COMPOUNDS AND USE THEREOF) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for RAPIVAB (Peramivir): An Industry Overview
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
