Expiring Drug Patents Cheat Sheet
We analyse the patents covering drugs in 134 countries and quickly give you the likely loss-of-exclusivity/generic entry date
Denmark: These 21 Drugs Face Patent Expirations and Generic Entry From 2025 - 2026
The content of this page is licensed under a Creative Commons Attribution 4.0 International License.

Generic Entry Dates in Other Countries
Friedman, Yali, "Denmark: These 21 Drugs Face Patent Expirations and Generic Entry From 2025 - 2026" DrugPatentWatch.com thinkBiotech, 2025 www.drugpatentwatch.com/p/expiring-drug-patents-generic-entry/.
Media collateral
These estimated drug patent expiration dates and generic entry opportunity dates are calculated from analysis of known patents covering drugs. Many factors can influence early or late generic entry. This information is provided as a rough estimate of generic entry potential and should not be used as an independent source. The methodology is described in this blog post.
When can CAMCEVI KIT (leuprolide mesylate) generic drug versions launch?
Generic name: leuprolide mesylate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: January 18, 2026
Generic Entry Controlled by: Denmark Patent 1,984,009
CAMCEVI KIT is a drug marketed by Accord. There are five patents protecting this drug.
This drug has thirty-nine patent family members in nineteen countries.
The generic ingredient in CAMCEVI KIT is leuprolide mesylate. There are twenty-two drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the leuprolide mesylate profile page.
When can DYANAVEL XR (amphetamine) generic drug versions launch?
Generic name: amphetamine
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: March 16, 2026
Generic Entry Controlled by: Denmark Patent 2,428,205
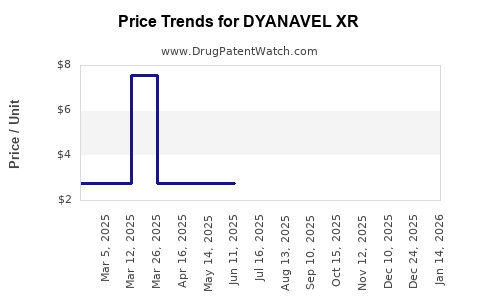
This drug has twenty-one patent family members in fourteen countries. There has been litigation on patents covering DYANAVEL XR
See drug price trends for DYANAVEL XR.
The generic ingredient in DYANAVEL XR is amphetamine. There are fifty-five drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the amphetamine profile page.
When can TUZISTRA XR (chlorpheniramine polistirex; codeine polistirex) generic drug versions launch?
Generic name: chlorpheniramine polistirex; codeine polistirex
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: March 16, 2026
Generic Entry Controlled by: Denmark Patent 2,428,205
TUZISTRA XR is a drug marketed by Tris Pharma Inc. There are two patents protecting this drug.
This drug has twenty-one patent family members in fourteen countries. There has been litigation on patents covering TUZISTRA XR
See drug price trends for TUZISTRA XR.
The generic ingredient in TUZISTRA XR is chlorpheniramine polistirex; codeine polistirex. There are twenty-nine drug master file entries for this API. Additional details are available on the chlorpheniramine polistirex; codeine polistirex profile page.
When can LASTACAFT (alcaftadine) generic drug versions launch?
Generic name: alcaftadine
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: March 31, 2026
Generic Entry Controlled by: Denmark Patent 2,004,196
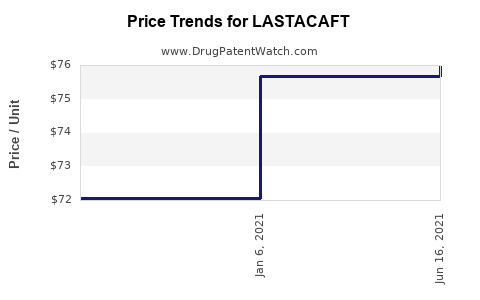
This drug has forty-six patent family members in thirty countries. There has been litigation on patents covering LASTACAFT
See drug price trends for LASTACAFT.
The generic ingredient in LASTACAFT is alcaftadine. There are six drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the alcaftadine profile page.
When can LASTACAFT (alcaftadine) generic drug versions launch?
Generic name: alcaftadine
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: March 31, 2026
Generic Entry Controlled by: Denmark Patent 3,150,209

This drug has forty-six patent family members in thirty countries. There has been litigation on patents covering LASTACAFT
See drug price trends for LASTACAFT.
The generic ingredient in LASTACAFT is alcaftadine. There are six drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the alcaftadine profile page.
When can TRADJENTA (linagliptin) generic drug versions launch?
Generic name: linagliptin
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: May 04, 2026
Generic Entry Controlled by: Denmark Patent 2,023,902
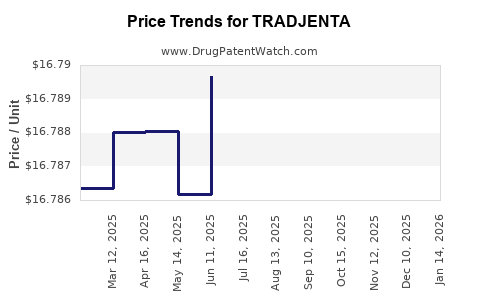
This drug has four hundred and eighty-six patent family members in forty-five countries. There has been litigation on patents covering TRADJENTA
See drug price trends for TRADJENTA.
The generic ingredient in TRADJENTA is linagliptin. There are nineteen drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the linagliptin profile page.
When can TRADJENTA (linagliptin) generic drug versions launch?
Generic name: linagliptin
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: May 04, 2026
Generic Entry Controlled by: Denmark Patent 2,277,509

This drug has four hundred and eighty-six patent family members in forty-five countries. There has been litigation on patents covering TRADJENTA
See drug price trends for TRADJENTA.
The generic ingredient in TRADJENTA is linagliptin. There are nineteen drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the linagliptin profile page.
When can TRADJENTA (linagliptin) generic drug versions launch?
Generic name: linagliptin
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: May 04, 2026
Generic Entry Controlled by: Denmark Patent 2,283,819

This drug has four hundred and eighty-six patent family members in forty-five countries. There has been litigation on patents covering TRADJENTA
See drug price trends for TRADJENTA.
The generic ingredient in TRADJENTA is linagliptin. There are nineteen drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the linagliptin profile page.
When can VANTRELA ER (hydrocodone bitartrate) generic drug versions launch?
Generic name: hydrocodone bitartrate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: June 15, 2026
Generic Entry Controlled by: Denmark Patent 2,200,593
VANTRELA ER is a drug marketed by Teva Branded Pharm. There are three patents protecting this drug. One tentatively approved generic is ready to enter the market.
This drug has thirty-three patent family members in thirteen countries. There has been litigation on patents covering VANTRELA ER
The generic ingredient in VANTRELA ER is hydrocodone bitartrate. There are twenty-three drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the hydrocodone bitartrate profile page.
When can TRINTELLIX (vortioxetine hydrobromide) generic drug versions launch?
Generic name: vortioxetine hydrobromide
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: June 16, 2026
Generic Entry Controlled by: Denmark Patent 2,044,043
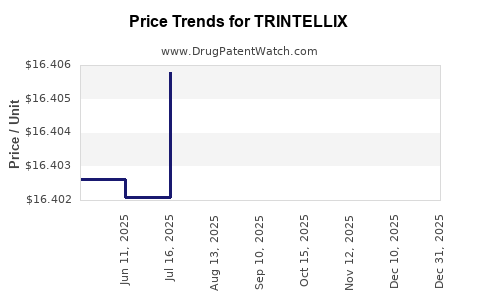
This drug has two hundred and seventeen patent family members in forty-two countries. There has been litigation on patents covering TRINTELLIX
See drug price trends for TRINTELLIX.
The generic ingredient in TRINTELLIX is vortioxetine hydrobromide. There are sixteen drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the vortioxetine hydrobromide profile page.
When can EMBEDA (morphine sulfate; naltrexone hydrochloride) generic drug versions launch?
Generic name: morphine sulfate; naltrexone hydrochloride
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: June 19, 2026
Generic Entry Controlled by: Denmark Patent 2,719,378
EMBEDA is a drug marketed by Alpharma Pharms. There are nine patents protecting this drug and four Paragraph IV challenges.
This drug has seventy-four patent family members in twenty-three countries.
See drug price trends for EMBEDA.
The generic ingredient in EMBEDA is morphine sulfate; naltrexone hydrochloride. There are twenty-three drug master file entries for this API. Additional details are available on the morphine sulfate; naltrexone hydrochloride profile page.
When can RASUVO (methotrexate) generic drug versions launch?
Generic name: methotrexate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: July 21, 2026
Generic Entry Controlled by: Denmark Patent 2,046,332
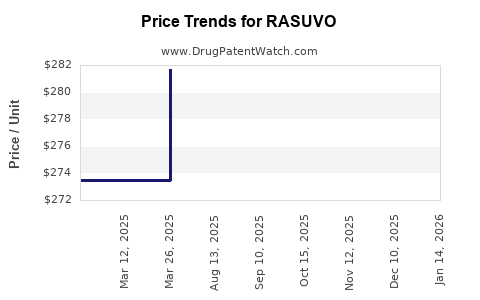
This drug has twenty-nine patent family members in twenty-one countries. There has been litigation on patents covering RASUVO
See drug price trends for RASUVO.
The generic ingredient in RASUVO is methotrexate. There are twenty drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the methotrexate profile page.
When can OLYSIO (simeprevir sodium) generic drug versions launch?
Generic name: simeprevir sodium
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: July 28, 2026
Generic Entry Controlled by: Denmark Patent 1,912,999
OLYSIO is a drug marketed by Janssen Prods. There are nine patents protecting this drug.
This drug has one hundred and forty patent family members in forty-three countries.
See drug price trends for OLYSIO.
The generic ingredient in OLYSIO is simeprevir sodium. There is one drug master file entry for this API. Additional details are available on the simeprevir sodium profile page.
When can CREON (pancrelipase (amylase;lipase;protease)) generic drug versions launch?
Generic name: pancrelipase (amylase;lipase;protease)
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: August 15, 2026
Generic Entry Controlled by: Denmark Patent 1,931,316
CREON is a drug marketed by
This drug has one hundred and forty patent family members in forty-three countries.
See drug price trends for CREON.
The generic ingredient in CREON is pancrelipase (amylase;lipase;protease). There are six drug master file entries for this API. Additional details are available on the pancrelipase (amylase;lipase;protease) profile page.
When can JYNARQUE (tolvaptan) generic drug versions launch?
Generic name: tolvaptan
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: September 01, 2026
Generic Entry Controlled by: Denmark Patent 1,919,874
JYNARQUE is a drug marketed by Otsuka. There are two patents protecting this drug.
This drug has eighty-six patent family members in twenty-four countries. There has been litigation on patents covering JYNARQUE
See drug price trends for JYNARQUE.
The generic ingredient in JYNARQUE is tolvaptan. There are eight drug master file entries for this API. Seven suppliers are listed for this generic product. Additional details are available on the tolvaptan profile page.
When can SAMSCA (tolvaptan) generic drug versions launch?
Generic name: tolvaptan
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: September 01, 2026
Generic Entry Controlled by: Denmark Patent 1,919,874
SAMSCA is a drug marketed by Otsuka. There are two patents protecting this drug and two Paragraph IV challenges.
This drug has eighty-six patent family members in twenty-four countries. There has been litigation on patents covering SAMSCA
See drug price trends for SAMSCA.
The generic ingredient in SAMSCA is tolvaptan. There are eight drug master file entries for this API. Seven suppliers are listed for this generic product. Additional details are available on the tolvaptan profile page.
When can ESBRIET (pirfenidone) generic drug versions launch?
Generic name: pirfenidone
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: September 22, 2026
Generic Entry Controlled by: Denmark Patent 1,940,364
ESBRIET is a drug marketed by Genentech Inc. There are twenty patents protecting this drug and two Paragraph IV challenges. One tentatively approved generic is ready to enter the market.
This drug has two hundred and sixty-six patent family members in forty-six countries. There has been litigation on patents covering ESBRIET
See drug price trends for ESBRIET.
The generic ingredient in ESBRIET is pirfenidone. There are twenty-three drug master file entries for this API. Twenty-four suppliers are listed for this generic product. Additional details are available on the pirfenidone profile page.
When can ZUNVEYL (benzgalantamine gluconate) generic drug versions launch?
Generic name: benzgalantamine gluconate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: September 22, 2026
Generic Entry Controlled by: Denmark Patent 1,940,817
ZUNVEYL is a drug marketed by Alpha Cognition. There are three patents protecting this drug.
This drug has twenty-six patent family members in seventeen countries. There has been litigation on patents covering ZUNVEYL
The generic ingredient in ZUNVEYL is benzgalantamine gluconate. One supplier is listed for this generic product. Additional details are available on the benzgalantamine gluconate profile page.
When can ENTRESTO SPRINKLE (sacubitril; valsartan) generic drug versions launch?
Generic name: sacubitril; valsartan
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: November 08, 2026
Generic Entry Controlled by: Denmark Patent 2,340,828
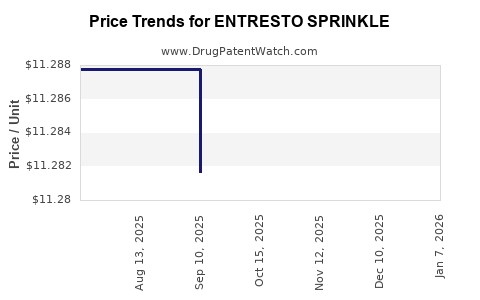
This drug has one hundred and forty-five patent family members in forty-three countries. There has been litigation on patents covering ENTRESTO SPRINKLE
See drug price trends for ENTRESTO SPRINKLE.
The generic ingredient in ENTRESTO SPRINKLE is sacubitril; valsartan. There are eleven drug master file entries for this API. Twenty-two suppliers are listed for this generic product. Additional details are available on the sacubitril; valsartan profile page.
When can XALKORI (crizotinib) generic drug versions launch?
Generic name: crizotinib
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: November 23, 2026
Generic Entry Controlled by: Denmark Patent 1,963,302
XALKORI is a drug marketed by Pf Prism Cv. There are five patents protecting this drug.
This drug has one hundred and fifty-two patent family members in forty-eight countries.
See drug price trends for XALKORI.
The generic ingredient in XALKORI is crizotinib. One supplier is listed for this generic product. Additional details are available on the crizotinib profile page.
When can SIRTURO (bedaquiline fumarate) generic drug versions launch?
Generic name: bedaquiline fumarate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: December 05, 2026
Generic Entry Controlled by: Denmark Patent 2,086,940
SIRTURO is a drug marketed by Janssen Therap. There are two patents protecting this drug.
This drug has ninety-seven patent family members in thirty-nine countries.
See drug price trends for SIRTURO.
The generic ingredient in SIRTURO is bedaquiline fumarate. There is one drug master file entry for this API. One supplier is listed for this generic product. Additional details are available on the bedaquiline fumarate profile page.
When can KOSELUGO (selumetinib sulfate) generic drug versions launch?
Generic name: selumetinib sulfate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: December 12, 2026
Generic Entry Controlled by: Denmark Patent 1,968,948
KOSELUGO is a drug marketed by Astrazeneca. There are eight patents protecting this drug.
This drug has two hundred and one patent family members in forty-five countries. There has been litigation on patents covering KOSELUGO
See drug price trends for KOSELUGO.
The generic ingredient in KOSELUGO is selumetinib sulfate. One supplier is listed for this generic product. Additional details are available on the selumetinib sulfate profile page.
When can LETAIRIS (ambrisentan) generic drug versions launch?
Generic name: ambrisentan
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: December 12, 2026
Generic Entry Controlled by: Denmark Patent 2,101,777
LETAIRIS is a drug marketed by Gilead. There are three patents protecting this drug and one Paragraph IV challenge.
This drug has fifty-one patent family members in twenty-six countries. There has been litigation on patents covering LETAIRIS
See drug price trends for LETAIRIS.
The generic ingredient in LETAIRIS is ambrisentan. There are nine drug master file entries for this API. Thirteen suppliers are listed for this generic product. Additional details are available on the ambrisentan profile page.
When can VYKAT XR (diazoxide choline) generic drug versions launch?
Generic name: diazoxide choline
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: December 20, 2026
Generic Entry Controlled by: Denmark Patent 1,968,601
VYKAT XR is a drug marketed by Soleno Therap. There are six patents protecting this drug.
This drug has seventy-eight patent family members in twenty-two countries.
The generic ingredient in VYKAT XR is diazoxide choline. There are five drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the diazoxide choline profile page.
Denmark Branded and Generic Drug Markets Assessment and Regulatory Opportunities and Challenges
More… ↓
DrugPatentWatch cited by CNN, NEJM, Nature Journals, and more …

Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
