VAFSEO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Vafseo, and when can generic versions of Vafseo launch?
Vafseo is a drug marketed by Akebia and is included in one NDA. There are thirteen patents protecting this drug.
This drug has two hundred and sixty-one patent family members in forty-eight countries.
The generic ingredient in VAFSEO is vadadustat. One supplier is listed for this compound. Additional details are available on the vadadustat profile page.
DrugPatentWatch® Generic Entry Outlook for Vafseo
Vafseo will be eligible for patent challenges on March 27, 2028. This date may extended up to six months if a pediatric exclusivity extension is applied to the drug's patents.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 27, 2029. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for VAFSEO?
- What are the global sales for VAFSEO?
- What is Average Wholesale Price for VAFSEO?
Summary for VAFSEO
| International Patents: | 261 |
| US Patents: | 13 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 1 |
| Patent Applications: | 247 |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for VAFSEO |
| What excipients (inactive ingredients) are in VAFSEO? | VAFSEO excipients list |
| DailyMed Link: | VAFSEO at DailyMed |
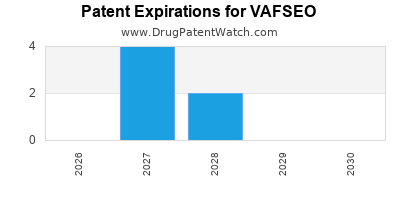
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for VAFSEO
Generic Entry Date for VAFSEO*:
Constraining patent/regulatory exclusivity:
NEW CHEMICAL ENTITY NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for VAFSEO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| USRC Kidney Research | PHASE3 |
| Akebia Therapeutics | PHASE3 |
Pharmacology for VAFSEO
US Patents and Regulatory Information for VAFSEO
VAFSEO is protected by thirteen US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of VAFSEO is ⤷ Get Started Free.
This potential generic entry date is based on NEW CHEMICAL ENTITY.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akebia | VAFSEO | vadadustat | TABLET;ORAL | 215192-003 | Mar 27, 2024 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Akebia | VAFSEO | vadadustat | TABLET;ORAL | 215192-003 | Mar 27, 2024 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Akebia | VAFSEO | vadadustat | TABLET;ORAL | 215192-002 | Mar 27, 2024 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Akebia | VAFSEO | vadadustat | TABLET;ORAL | 215192-003 | Mar 27, 2024 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for VAFSEO
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Akebia Europe Limited | Vafseo | vadadustat | EMEA/H/C/005131Vafseo is indicated for the treatment of symptomatic anaemia associated with chronic kidney disease (CKD) in adults on chronic maintenance dialysis. | Authorised | no | no | no | 2023-04-24 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for VAFSEO
When does loss-of-exclusivity occur for VAFSEO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 07265460
Patent: Prolyl hydroxylase inhibitors and methods of use
Estimated Expiration: ⤷ Get Started Free
Austria
Patent: 85264
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0713350
Patent: inibidores de prolil hidroxilase e métodos de utilização
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 59682
Patent: INHIBITEURS DE LA PROLYL HYDROXYLASE ET PROCEDES D'UTILISATION (PROLYL HYDROXYLASE INHIBITORS AND METHODS OF USE)
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1506149
Patent: Prolyl hydroxylase inhibitors and methods of use
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 70355
Patent: INHIBIDORES PROLIL HIDROXILASA Y METODOS DE USO
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 12021
Estimated Expiration: ⤷ Get Started Free
Patent: 25397
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 44005
Estimated Expiration: ⤷ Get Started Free
Patent: 26044
Estimated Expiration: ⤷ Get Started Free
Patent: 57911
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 44005
Patent: INHIBITEURS DE LA PROLYL HYDROXYLASE ET PROCÉDÉS D'UTILISATION (PROLYL HYDROXYLASE INHIBITORS AND METHODS OF USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 27696
Patent: Inhibiteurs de la prolyl hydroxylase et procédés d'utilisation (Prolyl hydroxylase inhibitors and methods of use)
Estimated Expiration: ⤷ Get Started Free
Patent: 26044
Patent: INHIBITEURS DE PROLYL HYDROXYLASE ET PROCÉDÉS D'UTILISATION (PROLYL HYDROXYLASE INHIBITORS AND METHODS OF USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 23807
Patent: INHIBITEURS DE PROLYL HYDROXYLASE ET PROCÉDÉS D'UTILISATION (PROLYL HYDROXYLASE INHIBITORS AND METHODS OF USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 57911
Patent: INHIBITEURS DE PROLYL HYDROXYLASE ET PROCÉDÉS D'UTILISATION (PROLYL HYDROXYLASE INHIBITORS AND METHODS OF USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 95127
Patent: INHIBITEURS DE PROLYL HYDROXYLASE ET PROCÉDÉS D'UTILISATION (PROLYL HYDROXYLASE INHIBITORS AND METHODS OF USE)
Estimated Expiration: ⤷ Get Started Free
Germany
Patent: 2007009992
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 29369
Patent: PROLYL HYDROXYLASE INHIBITORS AND METHODS OF USE
Estimated Expiration: ⤷ Get Started Free
Patent: 18548
Patent: 脯氨醯羥化酶抑制劑及其使用方法 (PROLYL HYDROXYLASE INHIBITORS AND METHODS OF USE)
Estimated Expiration: ⤷ Get Started Free
Patent: 58159
Patent: 脯氨醯羥化酶抑制劑及使用方法 (PROLYL HYDROXYLASE INHIBITORS AND METHODS OF USE)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 41300
Estimated Expiration: ⤷ Get Started Free
Patent: 59557
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 6127
Patent: מעכבי פרוליל הידרוקסילאז, מלחים שלהם המקובלים ברוקחות, תכשירי רוקחות המכילים אותם, ושימושים שלהם לטיפול במגוון מחלות (Prolyl hydroxylase inhibitors, pharmaceutically acceptable salts thereof, pharmaceutical compositions containing them and uses thereof for the treatment of a variety of diseases)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 13838
Estimated Expiration: ⤷ Get Started Free
Patent: 09541486
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 57911
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 09000286
Patent: INHIBIDORES DE PROLIL HIDROXILASA Y METODOS DE USO. (PROLYL HYDROXYLASE INHIBITORS AND METHODS OF USE.)
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 1730
Patent: Prolyl hydroxylase inhibitors and methods of use
Estimated Expiration: ⤷ Get Started Free
Patent: 1731
Patent: Prolyl hydroxylase inhibitors and methods for use
Estimated Expiration: ⤷ Get Started Free
Patent: 3002
Patent: Prolyl hydroxylase inhibitors and methods of use
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 44005
Estimated Expiration: ⤷ Get Started Free
Patent: 26044
Estimated Expiration: ⤷ Get Started Free
Patent: 57911
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 44005
Estimated Expiration: ⤷ Get Started Free
Patent: 26044
Estimated Expiration: ⤷ Get Started Free
Patent: 57911
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 29226
Patent: ИНГИБИТОРЫ ПРОЛИЛГИДРОКСИЛАЗЫ И СПОСОБЫ ИХ ПРИМЕНЕНИЯ (PROLYL HYDROXYLASE INHIBITORS AND APPLICATION METHODS THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 09102220
Patent: ИНГИБИТОРЫ ПРОЛИЛГИДРОКСИЛАЗЫ И СПОСОБЫ ИХ ПРИМЕНЕНИЯ
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 44005
Estimated Expiration: ⤷ Get Started Free
Patent: 57911
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1130592
Estimated Expiration: ⤷ Get Started Free
Patent: 090060264
Patent: PROLYL HYDROXYLASE INHIBITORS AND METHODS OF USE
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 54584
Estimated Expiration: ⤷ Get Started Free
Patent: 05587
Estimated Expiration: ⤷ Get Started Free
Patent: 22078
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1900548
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering VAFSEO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Peru | 20160194 | ⤷ Get Started Free | |
| Japan | 2020183408 | ⤷ Get Started Free | |
| European Patent Office | 2327696 | Inhibiteurs de la prolyl hydroxylase et procédés d'utilisation (Prolyl hydroxylase inhibitors and methods of use) | ⤷ Get Started Free |
| Japan | 2021191773 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VAFSEO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3007695 | PA2024521 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: VADADUSTATAS; REGISTRATION NO/DATE: EU/1/23/1725 20230424 |
| 3007695 | 2024C/524 | Belgium | ⤷ Get Started Free | PRODUCT NAME: VADADUSTAT; AUTHORISATION NUMBER AND DATE: EU/1/23/1725 20230425 |
| 3007695 | 24C1025 | France | ⤷ Get Started Free | PRODUCT NAME: VADADUSTAT; NAT. REGISTRATION NO/DATE: : EU/1/23/1725 20230425; FIRST REGISTRATION: EU/1/23/1725 20230425 |
| 3007695 | 122024000039 | Germany | ⤷ Get Started Free | PRODUCT NAME: VADADUSTAT (VAFSEO); REGISTRATION NO/DATE: EU/1/23/1725 20230424 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for the Pharmaceutical Drug: VAFSEO
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
