LYNPARZA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Lynparza, and what generic alternatives are available?
Lynparza is a drug marketed by Astrazeneca and is included in two NDAs. There are twelve patents protecting this drug and one Paragraph IV challenge.
This drug has two hundred and fifty-four patent family members in fifty-two countries.
The generic ingredient in LYNPARZA is olaparib. There are three drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the olaparib profile page.
DrugPatentWatch® Generic Entry Outlook for Lynparza
Lynparza was eligible for patent challenges on December 19, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be September 8, 2027. This may change due to patent challenges or generic licensing.
There have been seven patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are three tentative approvals for the generic drug (olaparib), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for LYNPARZA?
- What are the global sales for LYNPARZA?
- What is Average Wholesale Price for LYNPARZA?
Summary for LYNPARZA
| International Patents: | 254 |
| US Patents: | 12 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 97 |
| Clinical Trials: | 153 |
| Patent Applications: | 4,768 |
| Drug Prices: | Drug price information for LYNPARZA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for LYNPARZA |
| What excipients (inactive ingredients) are in LYNPARZA? | LYNPARZA excipients list |
| DailyMed Link: | LYNPARZA at DailyMed |
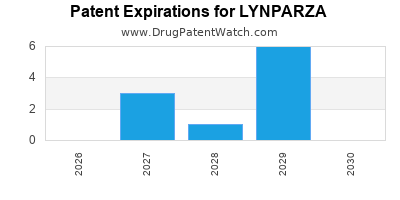

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for LYNPARZA
Generic Entry Dates for LYNPARZA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
Generic Entry Dates for LYNPARZA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for LYNPARZA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University Medical Center Groningen | Phase 4 |
| Pamela Munster | Phase 1 |
| Alexander B Olawaiye, MD | Phase 2 |
Pharmacology for LYNPARZA
| Drug Class | Poly(ADP-Ribose) Polymerase Inhibitor |
| Mechanism of Action | Poly(ADP-Ribose) Polymerase Inhibitors |
US Patents and Regulatory Information for LYNPARZA
LYNPARZA is protected by sixty US patents and five FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of LYNPARZA is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca | LYNPARZA | olaparib | TABLET;ORAL | 208558-001 | Aug 17, 2017 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Astrazeneca | LYNPARZA | olaparib | TABLET;ORAL | 208558-002 | Aug 17, 2017 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Astrazeneca | LYNPARZA | olaparib | TABLET;ORAL | 208558-001 | Aug 17, 2017 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for LYNPARZA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca | LYNPARZA | olaparib | TABLET;ORAL | 208558-002 | Aug 17, 2017 | ⤷ Get Started Free | ⤷ Get Started Free |
| Astrazeneca | LYNPARZA | olaparib | TABLET;ORAL | 208558-002 | Aug 17, 2017 | ⤷ Get Started Free | ⤷ Get Started Free |
| Astrazeneca | LYNPARZA | olaparib | TABLET;ORAL | 208558-002 | Aug 17, 2017 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for LYNPARZA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| AstraZeneca AB | Lynparza | olaparib | EMEA/H/C/003726Ovarian cancerLynparza is indicated as monotherapy for the:maintenance treatment of adult patients with advanced (FIGO stages III and IV) BRCA1/2-mutated (germline and/or somatic) high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) following completion of first-line platinum-based chemotherapy.maintenance treatment of adult patients with platinum sensitive relapsed high grade epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) to platinum based chemotherapy.Lynparza in combination with bevacizumab is indicated for the:maintenance treatment of adult patients with advanced (FIGO stages III and IV) high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) following completion of first-line platinum-based chemotherapy in combination with bevacizumab and whose cancer is associated with homologous recombination deficiency (HRD) positive status defined by either a BRCA1/2 mutation and/or genomic instability (see section 5.1).Breast cancerLynparza is indicated as:monotherapy or in combination with endocrine therapy for the adjuvant treatment of adult patients with germline BRCA1/2-mutations who have HER2-negative, high risk early breast cancer previously treated with neoadjuvant or adjuvant chemotherapy (see sections 4.2 and 5.1).monotherapy for the treatment of adult patients with germline BRCA1/2-mutations, who have HER2 negative locally advanced or metastatic breast cancer. Patients should have previously been treated with an anthracycline and a taxane in the (neo)adjuvant or metastatic setting unless patients were not suitable for these treatments (see section 5.1). Patients with hormone receptor (HR)-positive breast cancer should also have progressed on or after prior endocrine therapy, or be considered unsuitable for endocrine therapy.Adenocarcinoma of the pancreasLynparza is indicated as:monotherapy for the maintenance treatment of adult patients with germline BRCA1/2-mutations who have metastatic adenocarcinoma of the pancreas and have not progressed after a minimum of 16 weeks of platinum treatment within a first-line chemotherapy regimen.Prostate cancerLynparza is indicated as:monotherapy for the treatment of adult patients with metastatic castration-resistant prostate cancer (mCRPC) and BRCA1/2-mutations (germline and/or somatic) who have progressed following prior therapy that included a new hormonal agent.in combination with abiraterone and prednisone or prednisolone for the treatment of adult patients with mCRPC in whom chemotherapy is not clinically indicated (see section 5.1). | Authorised | no | no | no | 2014-12-16 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for LYNPARZA
When does loss-of-exclusivity occur for LYNPARZA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 3320
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 07311766
Estimated Expiration: ⤷ Get Started Free
Austria
Patent: 28296
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0717125
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 64275
Estimated Expiration: ⤷ Get Started Free
Patent: 75147
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 07002967
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1528714
Estimated Expiration: ⤷ Get Started Free
Patent: 2627611
Estimated Expiration: ⤷ Get Started Free
Patent: 4649979
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 10728
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0120007
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 12345
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 64189
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 64189
Estimated Expiration: ⤷ Get Started Free
Patent: 74800
Estimated Expiration: ⤷ Get Started Free
Patent: 24098
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 26483
Estimated Expiration: ⤷ Get Started Free
Patent: 03959
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 7420
Estimated Expiration: ⤷ Get Started Free
Patent: 6705
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 48513
Estimated Expiration: ⤷ Get Started Free
Patent: 07773
Estimated Expiration: ⤷ Get Started Free
Patent: 19471
Estimated Expiration: ⤷ Get Started Free
Patent: 10506894
Estimated Expiration: ⤷ Get Started Free
Patent: 13136607
Estimated Expiration: ⤷ Get Started Free
Patent: 15013879
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 7389
Estimated Expiration: ⤷ Get Started Free
Patent: 2829
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 09004103
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 987
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 5627
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 1963
Estimated Expiration: ⤷ Get Started Free
Patent: 3063
Estimated Expiration: ⤷ Get Started Free
Patent: 091882
Estimated Expiration: ⤷ Get Started Free
Patent: 171775
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 081175
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 64189
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 64189
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 65270
Estimated Expiration: ⤷ Get Started Free
Patent: 09109068
Estimated Expiration: ⤷ Get Started Free
Saudi Arabia
Patent: 280551
Estimated Expiration: ⤷ Get Started Free
Patent: 0310666
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 112
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 8523
Estimated Expiration: ⤷ Get Started Free
Patent: 201408404X
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 64189
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1494910
Estimated Expiration: ⤷ Get Started Free
Patent: 090085033
Estimated Expiration: ⤷ Get Started Free
Patent: 140011425
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 72630
Estimated Expiration: ⤷ Get Started Free
Patent: 87129
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 04716
Estimated Expiration: ⤷ Get Started Free
Patent: 0825066
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 494
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 639
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering LYNPARZA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Canada | 2875147 | ⤷ Get Started Free | |
| Eurasian Patent Organization | 009469 | ПРОИЗВОДНЫЕ ФТАЛАЗИНОНА (PHTHALAZINONE DERIVATIVES) | ⤷ Get Started Free |
| South Korea | 20060066067 | TRICYCLIC PARP INHIBITORS | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for LYNPARZA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1633724 | C300726 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: OLAPARIB, EN ZOUTEN EN; REGISTRATION NO/DATE: EU/1/14/959/001 20141216 |
| 2346495 | PA2018014 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: AMORFINIS OLAPARIBAS ARBA JO DRUSKA ARBA SOLVATAS KIETOJE DISPERSIJOJE; REGISTRATION NO/DATE: EU/1/14/959 20180508 |
| 1633724 | 13/2015 | Austria | ⤷ Get Started Free | PRODUCT NAME: OLAPARIB UND SALZE UND SOLVATE DAVON; REGISTRATION NO/DATE: EU/1/14/959 20141216 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for LYNPARZA (Olaparib)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
