BELSOMRA Drug Patent Profile
✉ Email this page to a colleague
When do Belsomra patents expire, and when can generic versions of Belsomra launch?
Belsomra is a drug marketed by Merck Sharp Dohme and is included in one NDA. There are three patents protecting this drug and one Paragraph IV challenge.
This drug has seventy-five patent family members in thirty-six countries.
The generic ingredient in BELSOMRA is suvorexant. One supplier is listed for this compound. Additional details are available on the suvorexant profile page.
DrugPatentWatch® Generic Entry Outlook for Belsomra
Belsomra was eligible for patent challenges on August 13, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be November 20, 2029. This may change due to patent challenges or generic licensing.
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for BELSOMRA?
- What are the global sales for BELSOMRA?
- What is Average Wholesale Price for BELSOMRA?
Summary for BELSOMRA
| International Patents: | 75 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 35 |
| Clinical Trials: | 34 |
| Patent Applications: | 495 |
| Drug Prices: | Drug price information for BELSOMRA |
| What excipients (inactive ingredients) are in BELSOMRA? | BELSOMRA excipients list |
| DailyMed Link: | BELSOMRA at DailyMed |
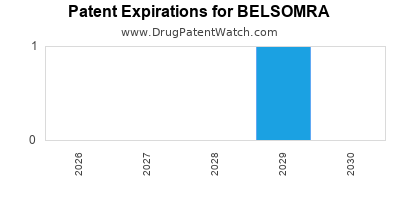
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for BELSOMRA
Generic Entry Date for BELSOMRA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for BELSOMRA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Massachusetts General Hospital | PHASE4 |
| Massachusetts Institute of Technology | PHASE4 |
| University of Maryland, Baltimore | Phase 2 |
Pharmacology for BELSOMRA
| Drug Class | Orexin Receptor Antagonist |
| Mechanism of Action | Cytochrome P450 3A Inhibitors Orexin Receptor Antagonists P-Glycoprotein Inhibitors |
Paragraph IV (Patent) Challenges for BELSOMRA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| BELSOMRA | Tablets | suvorexant | 5 mg, 10 mg, 15 mg and 20 mg | 204569 | 1 | 2024-03-04 |
US Patents and Regulatory Information for BELSOMRA
BELSOMRA is protected by three US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of BELSOMRA is ⤷ Get Started Free.
This potential generic entry date is based on patent 7,951,797.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Merck Sharp Dohme | BELSOMRA | suvorexant | TABLET;ORAL | 204569-001 | Aug 13, 2014 | RX | Yes | No | 7,951,797 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Merck Sharp Dohme | BELSOMRA | suvorexant | TABLET;ORAL | 204569-003 | Aug 13, 2014 | RX | Yes | No | 7,951,797 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Merck Sharp Dohme | BELSOMRA | suvorexant | TABLET;ORAL | 204569-002 | Aug 13, 2014 | RX | Yes | No | 7,951,797 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Merck Sharp Dohme | BELSOMRA | suvorexant | TABLET;ORAL | 204569-004 | Aug 13, 2014 | RX | Yes | Yes | 10,098,892 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Merck Sharp Dohme | BELSOMRA | suvorexant | TABLET;ORAL | 204569-003 | Aug 13, 2014 | RX | Yes | No | 11,980,623 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Merck Sharp Dohme | BELSOMRA | suvorexant | TABLET;ORAL | 204569-001 | Aug 13, 2014 | RX | Yes | No | 10,098,892 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for BELSOMRA
When does loss-of-exclusivity occur for BELSOMRA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 3979
Estimated Expiration: ⤷ Get Started Free
Patent: 8881
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 07328267
Estimated Expiration: ⤷ Get Started Free
Patent: 10249269
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0719361
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 70892
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 07003441
Estimated Expiration: ⤷ Get Started Free
Patent: 10001173
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1627028
Estimated Expiration: ⤷ Get Started Free
Patent: 1880276
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 90524
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 859
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0130002
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 13798
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 89382
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 009000126
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 099374
Estimated Expiration: ⤷ Get Started Free
El Salvador
Patent: 09003276
Patent: ANTAGONISTAS DE RECEPTOR DE OREXINA DE DIAZEPAN SUSTITUIDO
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 89382
Estimated Expiration: ⤷ Get Started Free
Patent: 92572
Estimated Expiration: ⤷ Get Started Free
Honduras
Patent: 09001067
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 28691
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 75427
Estimated Expiration: ⤷ Get Started Free
Patent: 35758
Estimated Expiration: ⤷ Get Started Free
Patent: 67803
Estimated Expiration: ⤷ Get Started Free
Patent: 10511621
Estimated Expiration: ⤷ Get Started Free
Patent: 11068665
Estimated Expiration: ⤷ Get Started Free
Patent: 11079848
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 1834
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 09005712
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 016
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 7334
Patent: SUBSTITUTED DIAZEPAN COMPOUNDS AS OREXIN RECEPTOR ANTAGONISTS
Estimated Expiration: ⤷ Get Started Free
Nicaragua
Patent: 0900100
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 2586
Estimated Expiration: ⤷ Get Started Free
Patent: 092470
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 081229
Patent: ANTAGONISTAS DE RECEPTOR DE OREXINA DE DIAZEPAM SUSTITUIDO
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 89382
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 89382
Estimated Expiration: ⤷ Get Started Free
Russian Federation
Patent: 58924
Patent: СОЕДИНЕНИЯ ЗАМЕЩЕННЫХ ДИАЗЕПАНОВ В КАЧЕСТВЕ АНТАГОНИСТОВ ОРЕКСИНОВЫХ РЕЦЕПТОРОВ (DIAZEPANE SUBSTITUTED COMPOUNDS AS OREXIN RECEPTOR ANTAGONISTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 61727
Patent: СОЕДИНЕНИЯ ЗАМЕЩЕННЫХ ДИАЗЕПАНОВ В КАЧЕСТВЕ АНТАГОНИСТОВ ОРЕКСИНОВЫХ РЕЦЕПТОРОВ (COMPOUNDS OF SUBSTITUTED DIAZEPANES AS ANTAGONISTS OF OREXIN RECEPTORS)
Estimated Expiration: ⤷ Get Started Free
Patent: 09125024
Patent: СОЕДИНЕНИЯ ЗАМЕЩЕННЫХ ДИАЗЕПАНОВ В КАЧЕСТВЕ АНТАГОНИСТОВ ОРЕКСИНОВЫХ РЕЦЕПТОРОВ
Estimated Expiration: ⤷ Get Started Free
Patent: 10150818
Patent: СОЕДИНЕНИЯ ЗАМЕЩЕННЫХ ДИАЗЕПАНОВ В КАЧЕСТВЕ АНТАГОНИСТОВ ОРЕКСИНОВЫХ РЕЦЕПТОРОВ
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 617
Patent: SUPSTITUISANA JEDINJENJA DIAZEPANA KAO ANTAGONISTI RECEPTORA ZA OREKSIN (SUBSTITUTED DIAZEPAN COMPOUNDS AS OREXIN RECEPTOR ANTAGONISTS)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 89382
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0903334
Patent: Substituted diazepan compounds as orexin receptor antagonists
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1217057
Estimated Expiration: ⤷ Get Started Free
Patent: 1299426
Estimated Expiration: ⤷ Get Started Free
Patent: 090087110
Estimated Expiration: ⤷ Get Started Free
Patent: 100031767
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 97188
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 13696
Estimated Expiration: ⤷ Get Started Free
Patent: 15188
Estimated Expiration: ⤷ Get Started Free
Patent: 0831494
Patent: Substituted diazepan orexin receptor antagonists
Estimated Expiration: ⤷ Get Started Free
Patent: 1109318
Patent: Substituted diazepan orexin receptor antagonists
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 0974
Patent: СОЕДИНЕНИЯ ЗАМЕЩЕННЫХ ДИАЗЕПАНОВ КАК АНТАГОНИСТЫ ОРЕКСИНОВЫХ РЕЦЕПТОРОВ;СПОЛУКИ ЗАМІЩЕНИХ ДІАЗЕПАНІВ ЯК АНТАГОНІСТИ ОРЕКСИНОВИХ РЕЦЕПТОРІВ (SUBSTITUTED DIAZEPAN COMPOUNDS AS OREXIN RECEPTOR ANTAGONISTS)
Estimated Expiration: ⤷ Get Started Free
Patent: 6873
Patent: СПОЛУКИ ЗАМІЩЕНИХ ДІАЗЕПАНІВ ЯК АНТАГОНІСТИ ОРЕКСИНОВИХ РЕЦЕПТОРІВ
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering BELSOMRA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Russian Federation | 2019126797 | СОСТАВЫ ТВЕРДЫХ ДОЗИРОВАННЫХ ЛЕКАРСТВЕННЫХ ФОРМ АНТАГОНИСТА ОРЕКСИНОВОГО РЕЦЕПТОРА | ⤷ Get Started Free |
| South Korea | 20090087110 | ⤷ Get Started Free | |
| Russian Federation | 2014152031 | СОСТАВЫ ТВЕРДЫХ ДОЗИРОВАННЫХ ЛЕКАРСТВЕННЫХ ФОРМ АНТАГОНИСТА ОРЕКСИНОВОГО РЕЦЕПТОРА | ⤷ Get Started Free |
| Japan | 6263169 | ⤷ Get Started Free | |
| Mexico | 368859 | FORMULACIONES DE DOSIS SOLIDA DE UN ANTAGONISTA DEL RECEPTOR DE OREXINA. (SOLID DOSAGE FORMULATIONS OF AN OREXIN RECEPTOR ANTAGONIST.) | ⤷ Get Started Free |
| Spain | 2397188 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for BELSOMRA (Suvorexant)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
