INVOKANA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Invokana, and when can generic versions of Invokana launch?
Invokana is a drug marketed by Janssen Pharms and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has two hundred and twenty patent family members in forty-five countries.
The generic ingredient in INVOKANA is canagliflozin. There are twenty-one drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the canagliflozin profile page.
DrugPatentWatch® Generic Entry Outlook for Invokana
Invokana was eligible for patent challenges on March 29, 2017.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be August 26, 2029. This may change due to patent challenges or generic licensing.
There have been twenty patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are five tentative approvals for the generic drug (canagliflozin), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for INVOKANA?
- What are the global sales for INVOKANA?
- What is Average Wholesale Price for INVOKANA?
Summary for INVOKANA
| International Patents: | 220 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 86 |
| Clinical Trials: | 24 |
| Patent Applications: | 1,277 |
| Drug Prices: | Drug price information for INVOKANA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for INVOKANA |
| What excipients (inactive ingredients) are in INVOKANA? | INVOKANA excipients list |
| DailyMed Link: | INVOKANA at DailyMed |
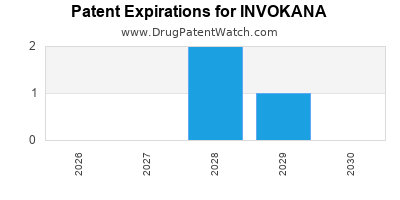
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for INVOKANA
Generic Entry Date for INVOKANA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for INVOKANA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Colorado, Denver | PHASE4 |
| Renal Pre-Competitive Consortium (RPC3) | PHASE4 |
| University of Michigan | PHASE4 |
Pharmacology for INVOKANA
| Drug Class | Sodium-Glucose Cotransporter 2 Inhibitor |
| Mechanism of Action | P-Glycoprotein Inhibitors Sodium-Glucose Transporter 2 Inhibitors |
Paragraph IV (Patent) Challenges for INVOKANA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| INVOKANA | Tablets | canagliflozin | 100 mg and 300 mg | 204042 | 10 | 2017-03-29 |
US Patents and Regulatory Information for INVOKANA
INVOKANA is protected by four US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of INVOKANA is ⤷ Get Started Free.
This potential generic entry date is based on patent 7,943,582.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen Pharms | INVOKANA | canagliflozin | TABLET;ORAL | 204042-001 | Mar 29, 2013 | RX | Yes | No | 7,943,582*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Janssen Pharms | INVOKANA | canagliflozin | TABLET;ORAL | 204042-002 | Mar 29, 2013 | RX | Yes | Yes | 7,943,582*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Janssen Pharms | INVOKANA | canagliflozin | TABLET;ORAL | 204042-001 | Mar 29, 2013 | RX | Yes | No | 8,513,202*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Janssen Pharms | INVOKANA | canagliflozin | TABLET;ORAL | 204042-002 | Mar 29, 2013 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Janssen Pharms | INVOKANA | canagliflozin | TABLET;ORAL | 204042-002 | Mar 29, 2013 | RX | Yes | Yes | 7,943,788*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for INVOKANA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Janssen-Cilag International NV | Invokana | canagliflozin | EMEA/H/C/002649Invokana is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise:as monotherapy when metformin is considered inappropriate due to intolerance or contraindicationsin addition to other medicinal products for the treatment of diabetes.For study results with respect to combination of therapies, effects on glycaemic control, cardiovascular and renal events, and the populations studied, see sections 4.4, 4.5 and 5.1. | Authorised | no | no | no | 2013-11-15 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for INVOKANA
When does loss-of-exclusivity occur for INVOKANA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 4099
Estimated Expiration: ⤷ Get Started Free
Patent: 7510
Estimated Expiration: ⤷ Get Started Free
Patent: 8450
Estimated Expiration: ⤷ Get Started Free
Patent: 9907
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 07329895
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0718882
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 71357
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 07003487
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1573368
Estimated Expiration: ⤷ Get Started Free
Patent: 2675299
Estimated Expiration: ⤷ Get Started Free
Patent: 2675380
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 10719
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 861
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0140254
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 14969
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 02224
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 099489
Estimated Expiration: ⤷ Get Started Free
El Salvador
Patent: 09003285
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 7103
Estimated Expiration: ⤷ Get Started Free
Patent: 0970540
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 02224
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 0900151
Estimated Expiration: ⤷ Get Started Free
Honduras
Patent: 09001135
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 9032
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 59788
Estimated Expiration: ⤷ Get Started Free
Patent: 10511602
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 3702
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 09005857
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 829
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 7545
Estimated Expiration: ⤷ Get Started Free
Nicaragua
Patent: 0900113
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 4354
Estimated Expiration: ⤷ Get Started Free
Patent: 091778
Estimated Expiration: ⤷ Get Started Free
Panama
Patent: 59401
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 081201
Estimated Expiration: ⤷ Get Started Free
Patent: 110841
Estimated Expiration: ⤷ Get Started Free
Patent: 130591
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 02224
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 02224
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 274
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 02224
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0903941
Patent: Crystalline form of 1- (Beta-D-Glucopyranosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl] benzene hemihydrate
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1146095
Estimated Expiration: ⤷ Get Started Free
Patent: 090086282
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 56640
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 03325
Estimated Expiration: ⤷ Get Started Free
Patent: 0829259
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 135
Patent: КРИСТАЛІЧНА ФОРМА ГЕМІГІДРАТУ 1-(β-D-ГЛЮКОПІРАНОЗИЛ)-4-МЕТИЛ-3-[5-(4-ФТОРФЕНІЛ)-2-ТІЕНІЛМЕТИЛ]БЕНЗОЛУ[КРИСТАЛЛИЧЕСКАЯ ФОРМА ГЕМИГИДРАТА 1-(b-D-ГЛЮКОПИРАНОЗИЛ)-4-МЕТИЛ-3-[5-(4-ФТОРФЕНИЛ)-2-ТИЭНИЛМЕТИЛ]БЕНЗОЛА (CRYSTALLINE FORM OF 1-(в-D-GLUCOPYRANOSYL)-4-METHYL-3-[5-(4-FLUOROPHENYL)-2-THIENYLMETHYL] BENZENE HEMIHYDRATE)
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 730
Patent: FORMA CRISTALINA DEL HEMIHIDRATO DE 1-(B (BETA)-D-GLUCOPIRANOSIL) -4-METIL-3-[5-(4-FLUOROFENIL) -2-TIENILMETIL]BENCENO
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering INVOKANA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Cyprus | 1119814 | ⤷ Get Started Free | |
| Eurasian Patent Organization | 011025 | НОВЫЕ СОЕДИНЕНИЯ (NOVEL COMPOUNDS) | ⤷ Get Started Free |
| Mexico | 2007009178 | DERIVADOS DE INDOL. (INDOLE DERIVATIVES.) | ⤷ Get Started Free |
| European Patent Office | 2568988 | COMPOSITIONS PHARMACEUTIQUES COMPRENANT DES DERIVES DE 1-(BETA-D-GLUCOPYRANOSYL)-2-THIENYLMETHYLBENZENE UTILES EN TANT QU'INHIBITEURS DE SGLT (PHARMACEUTICAL FORMULATIONS COMPRISING 1-(BETA-D-GLUCOPYRANOSYL)-2-THIENYLMETHYLBENZENE DERIVATIVES AS INHIBITORS OF SGLT) | ⤷ Get Started Free |
| South Korea | 20070100396 | INDOLE DERIVATIVES | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for INVOKANA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1651658 | C 2014 017 | Romania | ⤷ Get Started Free | PRODUCT NAME: CANAGLIFLOZIN; NATIONAL AUTHORISATION NUMBER: EU/1/13/884; DATE OF NATIONAL AUTHORISATION: 20131115; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/884; DATE OF FIRST AUTHORISATION IN EEA: 20131115 |
| 1651658 | 637 | Finland | ⤷ Get Started Free | |
| 1651658 | 164 1-2014 | Slovakia | ⤷ Get Started Free | PRODUCT NAME: KANAGLIFLOZIN; REGISTRATION NO/DATE: EU/1/13/884/001 - EU/1/13/884/008 20131115 |
| 1651658 | PA2014008,C1651658 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: CANAGLIFOZINUM; REGISTRATION NO/DATE: EU/1/13/884/001 - EU/1/13/884/004, 2013- 11-15 EU/1/13/884/005 - EU/1/13/884/008 20131115 |
| 1651658 | C300670 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: CANAGLIFLOZINE; REGISTRATION NO/DATE: EU/1/13/884/001-008 20131118 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
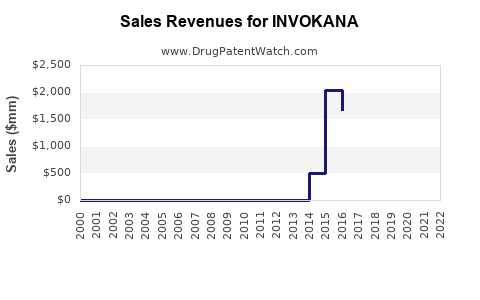
Market Dynamics and Financial Trajectory for INVOKANA (Canagliflozin)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
