CORLANOR Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Corlanor, and what generic alternatives are available?
Corlanor is a drug marketed by Amgen Inc and is included in two NDAs. There are eight patents protecting this drug and one Paragraph IV challenge.
This drug has ninety-seven patent family members in forty-two countries.
The generic ingredient in CORLANOR is ivabradine hydrochloride. There are nine drug master file entries for this compound. Eleven suppliers are listed for this compound. Additional details are available on the ivabradine hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Corlanor
A generic version of CORLANOR was approved as ivabradine hydrochloride by INGENUS PHARMS LLC on December 30th, 2021.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for CORLANOR?
- What are the global sales for CORLANOR?
- What is Average Wholesale Price for CORLANOR?
Summary for CORLANOR
| International Patents: | 97 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 84 |
| Clinical Trials: | 6 |
| Patent Applications: | 243 |
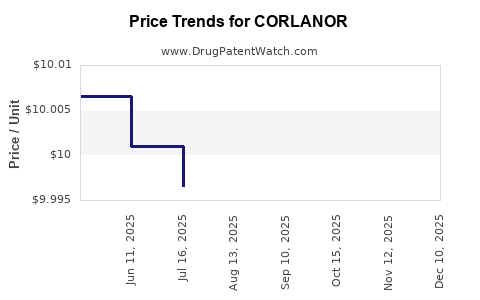
| Drug Prices: | Drug price information for CORLANOR |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for CORLANOR |
| What excipients (inactive ingredients) are in CORLANOR? | CORLANOR excipients list |
| DailyMed Link: | CORLANOR at DailyMed |
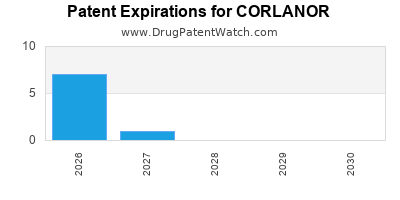
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for CORLANOR
Generic Entry Date for CORLANOR*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for CORLANOR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Amgen | Phase 3 |
| iRhythm Technologies, Inc. | Phase 4 |
| Phillip Levy | Phase 4 |
Pharmacology for CORLANOR
Paragraph IV (Patent) Challenges for CORLANOR
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| CORLANOR | Tablets | ivabradine hydrochloride | 5 mg and 7.5 mg | 206143 | 6 | 2019-10-15 |
US Patents and Regulatory Information for CORLANOR
CORLANOR is protected by eight US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of CORLANOR is ⤷ Get Started Free.
This potential generic entry date is based on patent 7,867,996.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amgen Inc | CORLANOR | ivabradine | SOLUTION;ORAL | 209964-001 | Apr 22, 2019 | RX | Yes | Yes | 7,867,996 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Amgen Inc | CORLANOR | ivabradine hydrochloride | TABLET;ORAL | 206143-001 | Apr 15, 2015 | AB | RX | Yes | No | 7,879,842*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Amgen Inc | CORLANOR | ivabradine | SOLUTION;ORAL | 209964-001 | Apr 22, 2019 | RX | Yes | Yes | 7,879,842 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for CORLANOR
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Accord Healthcare S.L.U. | Ivabradine Accord | ivabradine | EMEA/H/C/004241Symptomatic treatment of chronic stable angina pectorisIvabradine is indicated for the symptomatic treatment of chronic stable angina pectoris in coronary artery disease adults with normal sinus rhythm and heart rate ≥ 70 bpm.Ivabradine is indicated :- in adults unable to tolerate or with a contra-indication to the use of beta-blockers- or in combination with beta-blockers in patients inadequately controlled with an optimal beta-blocker dose.Treatment of chronic heart failureIvabradine is indicated in chronic heart failure NYHA II to IV class with systolic dysfunction, in patients in sinus rhythm and whose heart rate is ≥ 75 bpm, in combination with standard therapy including beta-blocker therapy or when beta-blocker therapy is contraindicated or not tolerated. (see section 5.1) | Authorised | yes | no | no | 2017-05-22 | |
| Zentiva, k.s. | Ivabradine Zentiva | ivabradine | EMEA/H/C/004117Symptomatic treatment of chronic stable angina pectoris Ivabradine is indicated for the symptomatic treatment of chronic stable angina pectoris in coronary artery disease adults with normal sinus rhythm and heart rate ≥ 70 bpm. Ivabradine is indicated:in adults unable to tolerate or with a contra-indication to the use of beta-blockersorin combination with beta-blockers in patients inadequately controlled with an optimal beta-blocker dose. Treatment of chronic heart failure Ivabradine is indicated in chronic heart failure NYHA II to IV class with systolic dysfunction, in patients in sinus rhythm and whose heart rate is ≥ 75 bpm, in combination with standard therapy including beta-blocker therapy or when beta-blocker therapy is contraindicated or not tolerated. | Authorised | yes | no | no | 2016-11-11 | |
| Les Laboratoires Servier | Corlentor | ivabradine | EMEA/H/C/000598Symptomatic treatment of chronic stable angina pectorisIvabradine is indicated for the symptomatic treatment of chronic stable angina pectoris in coronary artery disease adults with normal sinus rhythm and heart rate ≥ 70 bpm. Ivabradine is indicated:in adults unable to tolerate or with a contraindication to the use of beta-blockersor in combination with beta-blockers in patients inadequately controlled with an optimal beta-blocker dose.Treatment of chronic heart failureIvabradine is indicated in chronic heart failure NYHA II to IV class with systolic dysfunction, in patients in sinus rhythm and whose heart rate is ≥ 75 bpm, in combination with standard therapy including beta-blocker therapy or when beta-blocker therapy is contraindicated or not tolerated. | Authorised | no | no | no | 2005-10-25 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for CORLANOR
When does loss-of-exclusivity occur for CORLANOR?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 07
Patent: Beta-crystalline form of ivabradine hydrochloride,a process for its preparation and pharmaceutical compositions containing it
Estimated Expiration: ⤷ Get Started Free
Patent: 56
Patent: y-Crystalline form of Ivabradine Hydrochloride, a process for its preperation and pharmaceutical compositions containing it
Estimated Expiration: ⤷ Get Started Free
Argentina
Patent: 2926
Patent: FORMA CRISTALINA GAMA DEL CLORHIDRATO DE IVABRADINA, UN PROCEDIMIENTO PARA SU PREPARACION Y COMPOSICIONES QUE LA CONTIENEN
Estimated Expiration: ⤷ Get Started Free
Patent: 3147
Patent: FORMA CRISTALINA DEL CLORHIDRATO DE IVABRADINA, UN PROCEDIMIENTO PARA SU PREPARACION Y COMPOSICIONES FARMACEUTICAS QUE LA CONTIENEN
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 37400
Patent: FORME CRISTALLINE Y DU CHLORHYDRATE DE L'IVABRADINE, SON PROCEDE DE PREPARATION, ET LES COMPOSITIONS PHARMACEUTIQUES QUI LA CONTIENNENT (Y CRYSTALLINE FORM OF IVABRADINE CHLORHYDRATE, PROCESS FOR THE PREPARATION THEREOF AND PHARMACEUTICAL COMPOUNDS CONTAINING IT)
Estimated Expiration: ⤷ Get Started Free
Patent: 37414
Patent: FORME CRISTALLINE B DU CHLORHYDRATE DE L'IVABRADINE, SON PROCEDE DE PREPARATION, ET LES COMPOSITIONS PHARMACEUTIQUES QUI LA CONTIENNENT (B CRYSTALLINE FORM OF IVABRADINE CHLORHYDRATE, PROCESS FOR THE PREPARATION THEREOF AND PHARMACEUTICAL COMPOUNDS CONTAINING IT)
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 8128
Patent: B-CRYSTALLINE FORM OF IVABRADINE HYDROCHLORIDE, A PROCESS FOR ITS PREPARATION AND PHARMACEUTICAL COMPOSITIONS CONTAINING IT
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 5228
Patent: Beta-crystalline form of ivabradine hydrochloride,a process for its preparation and pharmaceutical compositions containing it
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering CORLANOR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 1695965 | Forme cristalline beta du chlorhydrate de l'ivabradine, son procédé de préparation, et les compositions pharmaceutiques qui la contiennent (Crystalline form beta of the chlorhydrate of ivabradine, process for its preparation and pharamcetuical composition containing it) | ⤷ Get Started Free |
| Ecuador | SP066375 | FORMA CRISTALINA GAMMA DE CLORHIDRATO DE IVABRADINA, SU PROCEDIMIENTO DE PREPARACIÓN Y COMPOSICIONES FARMACÉUTICAS QUE LA CONTIENEN | ⤷ Get Started Free |
| Portugal | 1695965 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Market Dynamics and Financial Trajectory for CORLANOR (Ivabradine)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
