United States: These 39 Drugs Face Patent Expirations and Generic Entry From 2024 - 2025
DrugPatentWatch® Estimated Loss of Exclusivity Dates in the United States
The content of this page is licensed under a Creative Commons Attribution 4.0 International License.

Generic Entry Dates in Other Countries
Friedman, Yali, "United States: These 39 Drugs Face Patent Expirations and Generic Entry From 2024 - 2025" DrugPatentWatch.com thinkBiotech, 2024 www.drugpatentwatch.com/p/expiring-drug-patents-generic-entry/.
Media collateral
These estimated drug patent expiration dates and generic entry opportunity dates are calculated from analysis of known patents covering drugs. Many factors can influence early or late generic entry. This information is provided as a rough estimate of generic entry potential and should not be used as an independent source. The methodology is described in this blog post.
When can KIMYRSA (oritavancin diphosphate) generic drug versions launch?
Generic name: oritavancin diphosphate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: August 06, 2024
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
KIMYRSA is a drug marketed by Melinta Therap. There are three patents protecting this drug.
This drug has thirty-nine patent family members in seventeen countries.
See drug price trends for KIMYRSA.
The generic ingredient in KIMYRSA is oritavancin diphosphate. One supplier is listed for this generic product. Additional details are available on the oritavancin diphosphate profile page.
When can ORBACTIV (oritavancin diphosphate) generic drug versions launch?
Generic name: oritavancin diphosphate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: August 06, 2024
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
ORBACTIV is a drug marketed by Melinta Therap. There are three patents protecting this drug.
This drug has thirty-nine patent family members in seventeen countries.
See drug price trends for ORBACTIV.
The generic ingredient in ORBACTIV is oritavancin diphosphate. One supplier is listed for this generic product. Additional details are available on the oritavancin diphosphate profile page.
When can SOMATULINE DEPOT (lanreotide acetate) generic drug versions launch?
Generic name: lanreotide acetate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: September 15, 2024
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
SOMATULINE DEPOT is a drug marketed by Ipsen Pharma.
This drug has thirty-nine patent family members in seventeen countries.
The generic ingredient in SOMATULINE DEPOT is lanreotide acetate. There are three drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the lanreotide acetate profile page.
When can SUSTOL (granisetron) generic drug versions launch?
Generic name: granisetron
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: September 28, 2024
Generic Entry Controlled by: United States Patent Patent 8,252,304
SUSTOL is a drug marketed by Heron Theraps Inc. There are five patents protecting this drug.
This drug has eighteen patent family members in ten countries.
See drug price trends for SUSTOL.
The generic ingredient in SUSTOL is granisetron. There are twenty-six drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the granisetron profile page.
When can PRIALT (ziconotide acetate) generic drug versions launch?
Generic name: ziconotide acetate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: October 01, 2024
Generic Entry Controlled by: United States Patent Patent 5,364,842
PRIALT is a drug marketed by Tersera. There are three patents protecting this drug.
This drug has six patent family members in four countries.
See drug price trends for PRIALT.
The generic ingredient in PRIALT is ziconotide acetate. There is one drug master file entry for this API. One supplier is listed for this generic product. Additional details are available on the ziconotide acetate profile page.
When can ASCOR (ascorbic acid) generic drug versions launch?
Generic name: ascorbic acid
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: October 02, 2024
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
ASCOR is a drug marketed by Mcguff.
This drug has six patent family members in four countries.
See drug price trends for ASCOR.
The generic ingredient in ASCOR is ascorbic acid. There are six drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the ascorbic acid profile page.
When can XOPENEX HFA (levalbuterol tartrate) generic drug versions launch?
Generic name: levalbuterol tartrate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: October 08, 2024
Generic Entry Controlled by: United States Patent Patent 7,256,310
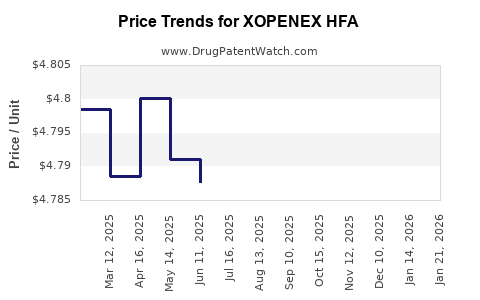
This drug has thirty-six patent family members in eighteen countries. There has been litigation on patents covering XOPENEX HFA
See drug price trends for XOPENEX HFA.
The generic ingredient in XOPENEX HFA is levalbuterol tartrate. There are nine drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the levalbuterol tartrate profile page.
When can FLUORODOPA F18 (fluorodopa f-18) generic drug versions launch?
Generic name: fluorodopa f-18
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: October 10, 2024
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
FLUORODOPA F18 is a drug marketed by Feinstein.
This drug has thirty-six patent family members in eighteen countries.
The generic ingredient in FLUORODOPA F18 is fluorodopa f-18. One supplier is listed for this generic product. Additional details are available on the fluorodopa f-18 profile page.
When can BYETTA (exenatide synthetic) generic drug versions launch?
Generic name: exenatide synthetic
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: November 04, 2024
Generic Entry Controlled by: United States FDA Regulatory Exclusivity

This drug has thirty-six patent family members in eighteen countries. There has been litigation on patents covering BYETTA
See drug price trends for BYETTA.
The generic ingredient in BYETTA is exenatide synthetic. There are seven drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the exenatide synthetic profile page.
When can EXEM FOAM KIT (air polymer-type a) generic drug versions launch?
Generic name: air polymer-type a
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: November 07, 2024
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
EXEM FOAM KIT is a drug marketed by Giskit. There are three patents protecting this drug.
This drug has six patent family members in six countries.
The generic ingredient in EXEM FOAM KIT is air polymer-type a. There are thirty-seven drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the air polymer-type a profile page.
When can NOURIANZ (istradefylline) generic drug versions launch?
Generic name: istradefylline
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: November 13, 2024
Generic Entry Controlled by: United States Patent Patent 7,541,363
NOURIANZ is a drug marketed by Kyowa Kirin. There are three patents protecting this drug.
This drug has sixty-three patent family members in eighteen countries.
See drug price trends for NOURIANZ.
The generic ingredient in NOURIANZ is istradefylline. There is one drug master file entry for this API. One supplier is listed for this generic product. Additional details are available on the istradefylline profile page.
When can MACRILEN (macimorelin acetate) generic drug versions launch?
Generic name: macimorelin acetate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: December 20, 2024
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
MACRILEN is a drug marketed by Novo. There is one patent protecting this drug.
This drug has eleven patent family members in ten countries.
See drug price trends for MACRILEN.
The generic ingredient in MACRILEN is macimorelin acetate. One supplier is listed for this generic product. Additional details are available on the macimorelin acetate profile page.
When can RYALTRIS (mometasone furoate; olopatadine hydrochloride) generic drug versions launch?
Generic name: mometasone furoate; olopatadine hydrochloride
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: January 13, 2025
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
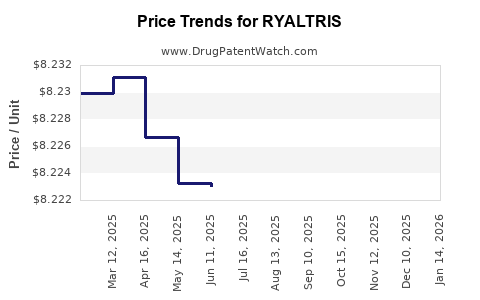
This drug has eighty-eight patent family members in thirty-two countries.
See drug price trends for RYALTRIS.
The generic ingredient in RYALTRIS is mometasone furoate; olopatadine hydrochloride. There are thirty drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the mometasone furoate; olopatadine hydrochloride profile page.
When can SANCUSO (granisetron) generic drug versions launch?
Generic name: granisetron
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: January 22, 2025
Generic Entry Controlled by: United States Patent Patent 7,608,282
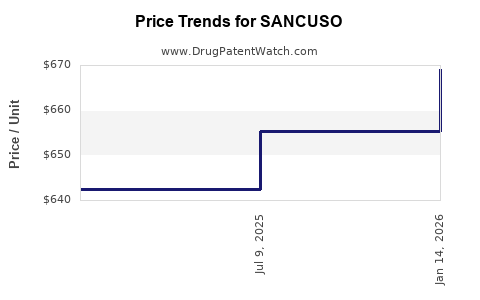
This drug has forty patent family members in thirty-one countries. There has been litigation on patents covering SANCUSO
See drug price trends for SANCUSO.
The generic ingredient in SANCUSO is granisetron. There are twenty-six drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the granisetron profile page.
When can DUAKLIR PRESSAIR (aclidinium bromide; formoterol fumarate) generic drug versions launch?
Generic name: aclidinium bromide; formoterol fumarate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: February 10, 2025
Generic Entry Controlled by: United States Patent Patent RE46417
DUAKLIR PRESSAIR is a drug marketed by Covis. There are four patents protecting this drug.
This drug has one hundred and fifty-six patent family members in forty-six countries.
See drug price trends for DUAKLIR PRESSAIR.
The generic ingredient in DUAKLIR PRESSAIR is aclidinium bromide; formoterol fumarate. There is one drug master file entry for this API. Two suppliers are listed for this generic product. Additional details are available on the aclidinium bromide; formoterol fumarate profile page.
When can TUDORZA PRESSAIR (aclidinium bromide) generic drug versions launch?
Generic name: aclidinium bromide
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: February 10, 2025
Generic Entry Controlled by: United States Patent Patent RE46417
TUDORZA PRESSAIR is a drug marketed by Covis. There are four patents protecting this drug.
This drug has one hundred and fifty-six patent family members in forty-six countries.
See drug price trends for TUDORZA PRESSAIR.
The generic ingredient in TUDORZA PRESSAIR is aclidinium bromide. There is one drug master file entry for this API. Two suppliers are listed for this generic product. Additional details are available on the aclidinium bromide profile page.
When can PIZENSY (lactitol) generic drug versions launch?
Generic name: lactitol
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: February 12, 2025
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
PIZENSY is a drug marketed by Braintree Labs. There is one patent protecting this drug.
The generic ingredient in PIZENSY is lactitol. There are four drug master file entries for this API. Additional details are available on the lactitol profile page.
When can IZERVAY (avacincaptad pegol sodium) generic drug versions launch?
Generic name: avacincaptad pegol sodium
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: February 14, 2025
Generic Entry Controlled by: United States Patent Patent 10,947,544
IZERVAY is a drug marketed by Astellas. There are eight patents protecting this drug.
This drug has one hundred patent family members in twenty-seven countries.
See drug price trends for IZERVAY.
The generic ingredient in IZERVAY is avacincaptad pegol sodium. One supplier is listed for this generic product. Additional details are available on the avacincaptad pegol sodium profile page.
When can IXEMPRA KIT (ixabepilone) generic drug versions launch?
Generic name: ixabepilone
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: February 21, 2025
Generic Entry Controlled by: United States Patent Patent 7,312,237
IXEMPRA KIT is a drug marketed by R-pharm Us Llc. There is one patent protecting this drug and one Paragraph IV challenge.
This drug has thirty-four patent family members in twenty-six countries.
See drug price trends for IXEMPRA KIT.
The generic ingredient in IXEMPRA KIT is ixabepilone. There is one drug master file entry for this API. One supplier is listed for this generic product. Additional details are available on the ixabepilone profile page.
When can BARHEMSYS (amisulpride) generic drug versions launch?
Generic name: amisulpride
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: February 26, 2025
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
BARHEMSYS is a drug marketed by Acacia. There are five patents protecting this drug.
This drug has sixty-eight patent family members in twenty-seven countries.
See drug price trends for BARHEMSYS.
The generic ingredient in BARHEMSYS is amisulpride. There are four drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the amisulpride profile page.
When can XARELTO (rivaroxaban) generic drug versions launch?
Generic name: rivaroxaban
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: February 28, 2025
Generic Entry Controlled by: United States Patent Patent 7,157,456
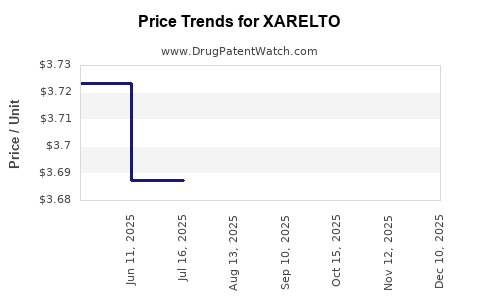
This drug has one hundred and fifty-six patent family members in forty-seven countries. There has been litigation on patents covering XARELTO
See drug price trends for XARELTO.
The generic ingredient in XARELTO is rivaroxaban. There are thirty-five drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the rivaroxaban profile page.
When can JUXTAPID (lomitapide mesylate) generic drug versions launch?
Generic name: lomitapide mesylate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: March 07, 2025
Generic Entry Controlled by: United States Patent Patent 8,618,135
JUXTAPID is a drug marketed by Chiesi. There are eight patents protecting this drug.
This drug has twenty-seven patent family members in eighteen countries. There has been litigation on patents covering JUXTAPID
See drug price trends for JUXTAPID.
The generic ingredient in JUXTAPID is lomitapide mesylate. There are two drug master file entries for this API. Three suppliers are listed for this generic product. Additional details are available on the lomitapide mesylate profile page.
When can SMOFLIPID 20% (fish oil; medium chain triglycerides; olive oil; soybean oil) generic drug versions launch?
Generic name: fish oil; medium chain triglycerides; olive oil; soybean oil
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: March 22, 2025
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
SMOFLIPID 20% is a drug marketed by Fresenius Kabi Usa.
This drug has twenty-seven patent family members in eighteen countries.
The generic ingredient in SMOFLIPID 20% is fish oil; medium chain triglycerides; olive oil; soybean oil. There are six drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the fish oil; medium chain triglycerides; olive oil; soybean oil profile page.
When can XELSTRYM (dextroamphetamine) generic drug versions launch?
Generic name: dextroamphetamine
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: March 22, 2025
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
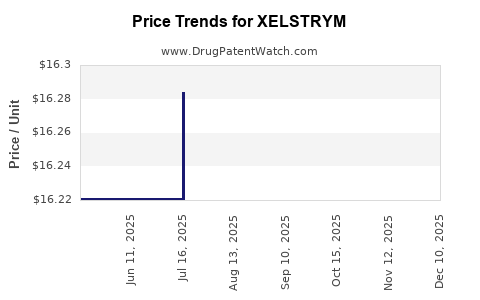
This drug has thirty-three patent family members in twelve countries. There has been litigation on patents covering XELSTRYM
See drug price trends for XELSTRYM.
The generic ingredient in XELSTRYM is dextroamphetamine. There are eighteen drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the dextroamphetamine profile page.
When can TLANDO (testosterone undecanoate) generic drug versions launch?
Generic name: testosterone undecanoate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: March 28, 2025
Generic Entry Controlled by: United States FDA Regulatory Exclusivity

This drug has thirty-six patent family members in fifteen countries.
See drug price trends for TLANDO.
The generic ingredient in TLANDO is testosterone undecanoate. There are sixty-nine drug master file entries for this API. Five suppliers are listed for this generic product. Additional details are available on the testosterone undecanoate profile page.
When can TARGINIQ (naloxone hydrochloride; oxycodone hydrochloride) generic drug versions launch?
Generic name: naloxone hydrochloride; oxycodone hydrochloride
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: March 30, 2025
Generic Entry Controlled by: United States Patent Patent 9,522,919
TARGINIQ is a drug marketed by Purdue Pharma Lp. There are two patents protecting this drug.
This drug has one hundred and fifty-one patent family members in forty-one countries. There has been litigation on patents covering TARGINIQ
The generic ingredient in TARGINIQ is naloxone hydrochloride; oxycodone hydrochloride. There are twelve drug master file entries for this API. Additional details are available on the naloxone hydrochloride; oxycodone hydrochloride profile page.
When can EDURANT PED (rilpivirine hydrochloride) generic drug versions launch?
Generic name: rilpivirine hydrochloride
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: April 21, 2025
Generic Entry Controlled by: United States Patent Patent 7,125,879
EDURANT PED is a drug marketed by Janssen Prods. There are two patents protecting this drug.
This drug has two hundred and twenty-one patent family members in forty-five countries. There has been litigation on patents covering EDURANT PED
The generic ingredient in EDURANT PED is rilpivirine hydrochloride. There are seven drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the rilpivirine hydrochloride profile page.
When can XARELTO (rivaroxaban) generic drug versions launch?
Generic name: rivaroxaban
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: May 13, 2025
Generic Entry Controlled by: United States Patent Patent 9,415,053

This drug has one hundred and fifty-six patent family members in forty-seven countries. There has been litigation on patents covering XARELTO
See drug price trends for XARELTO.
The generic ingredient in XARELTO is rivaroxaban. There are thirty-five drug master file entries for this API. Four suppliers are listed for this generic product. Additional details are available on the rivaroxaban profile page.
When can CERIANNA (fluoroestradiol f-18) generic drug versions launch?
Generic name: fluoroestradiol f-18
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: May 20, 2025
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
CERIANNA is a drug marketed by Ge Healthcare.
This drug has one hundred and fifty-six patent family members in forty-seven countries.
The generic ingredient in CERIANNA is fluoroestradiol f-18. One supplier is listed for this generic product. Additional details are available on the fluoroestradiol f-18 profile page.
When can FOLOTYN (pralatrexate) generic drug versions launch?
Generic name: pralatrexate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: May 31, 2025
Generic Entry Controlled by: United States Patent Patent 8,299,078
FOLOTYN is a drug marketed by Acrotech Biopharma. There are two patents protecting this drug and one Paragraph IV challenge. One tentatively approved generic is ready to enter the market.
This drug has thirty-one patent family members in twenty-three countries. There has been litigation on patents covering FOLOTYN
See drug price trends for FOLOTYN.
The generic ingredient in FOLOTYN is pralatrexate. There are two drug master file entries for this API. Two suppliers are listed for this generic product. Additional details are available on the pralatrexate profile page.
When can ABLYSINOL (alcohol) generic drug versions launch?
Generic name: alcohol
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: June 21, 2025
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
ABLYSINOL is a drug marketed by Bpi Labs.
This drug has thirty-one patent family members in twenty-three countries.
The generic ingredient in ABLYSINOL is alcohol. There are forty-one drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the alcohol profile page.
When can QSYMIA (phentermine hydrochloride; topiramate) generic drug versions launch?
Generic name: phentermine hydrochloride; topiramate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: June 24, 2025
Generic Entry Controlled by: United States FDA Regulatory Exclusivity

This drug has forty patent family members in seventeen countries. There has been litigation on patents covering QSYMIA
See drug price trends for QSYMIA.
The generic ingredient in QSYMIA is phentermine hydrochloride; topiramate. There are seventeen drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the phentermine hydrochloride; topiramate profile page.
When can NUCYNTA ER (tapentadol hydrochloride) generic drug versions launch?
Generic name: tapentadol hydrochloride
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: June 27, 2025
Generic Entry Controlled by: United States Patent Patent 7,994,364

This drug has two hundred and seventy-two patent family members in thirty-one countries. There has been litigation on patents covering NUCYNTA ER
See drug price trends for NUCYNTA ER.
The generic ingredient in NUCYNTA ER is tapentadol hydrochloride. There are five drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the tapentadol hydrochloride profile page.
When can TROXYCA ER (naltrexone hydrochloride; oxycodone hydrochloride) generic drug versions launch?
Generic name: naltrexone hydrochloride; oxycodone hydrochloride
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: July 03, 2025
Generic Entry Controlled by: United States Patent Patent 8,685,443
TROXYCA ER is a drug marketed by Pfizer. There are two patents protecting this drug.
This drug has sixteen patent family members in twelve countries.
The generic ingredient in TROXYCA ER is naltrexone hydrochloride; oxycodone hydrochloride. There are nineteen drug master file entries for this API. Additional details are available on the naltrexone hydrochloride; oxycodone hydrochloride profile page.
When can KRINTAFEL (tafenoquine succinate) generic drug versions launch?
Generic name: tafenoquine succinate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: July 20, 2025
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
KRINTAFEL is a drug marketed by Glaxosmithkline.
This drug has sixteen patent family members in twelve countries.
See drug price trends for KRINTAFEL.
The generic ingredient in KRINTAFEL is tafenoquine succinate. Two suppliers are listed for this generic product. Additional details are available on the tafenoquine succinate profile page.
When can KYZATREX (testosterone undecanoate) generic drug versions launch?
Generic name: testosterone undecanoate
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: July 27, 2025
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
KYZATREX is a drug marketed by Marius Pharms Llc. There are four patents protecting this drug. One tentatively approved generic is ready to enter the market.
This drug has twenty patent family members in eleven countries.
See drug price trends for KYZATREX.
The generic ingredient in KYZATREX is testosterone undecanoate. There are sixty-nine drug master file entries for this API. Five suppliers are listed for this generic product. Additional details are available on the testosterone undecanoate profile page.
When can OMEGAVEN (fish oil triglycerides) generic drug versions launch?
Generic name: fish oil triglycerides
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: July 27, 2025
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
OMEGAVEN is a drug marketed by Fresenius Kabi Usa. There are three patents protecting this drug.
This drug has five patent family members in four countries. There has been litigation on patents covering OMEGAVEN
See drug price trends for OMEGAVEN.
The generic ingredient in OMEGAVEN is fish oil triglycerides. There are six drug master file entries for this API. One supplier is listed for this generic product. Additional details are available on the fish oil triglycerides profile page.
When can AZEDRA (iobenguane i-131) generic drug versions launch?
Generic name: iobenguane i-131
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: July 30, 2025
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
AZEDRA is a drug marketed by Progenics Pharms Inc.
This drug has five patent family members in four countries.
See drug price trends for AZEDRA.
The generic ingredient in AZEDRA is iobenguane i-131. Additional details are available on the iobenguane i-131 profile page.
When can GALAFOLD (migalastat hydrochloride) generic drug versions launch?
Generic name: migalastat hydrochloride
DrugPatentWatch® Estimated Key Patent Expiration / Generic Entry Date: August 10, 2025
Generic Entry Controlled by: United States FDA Regulatory Exclusivity
GALAFOLD is a drug marketed by Amicus Therap Us. There are fifty-eight patents protecting this drug.
This drug has two hundred and thirty-three patent family members in thirty countries. There has been litigation on patents covering GALAFOLD
The generic ingredient in GALAFOLD is migalastat hydrochloride. One supplier is listed for this generic product. Additional details are available on the migalastat hydrochloride profile page.
DrugPatentWatch cited by CNN, NEJM, Nature Journals, and more …

Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


