|
4.3/5 based on G2 reviews |
4.4/5 based on Crozdesk reviews |
DrugPatentWatch cited by CNN, NEJM, Nature Journals, and more …

Don’t just take our word for it.
See why others use DrugPatentWatch and recommend it so highly.
❝ Information you don´t find anywhere else ❞
❝ I like best … the flexible subscription plans (by module, by month, by year, etc.) ❞
❝ My overall experience with DPW is very positive. The team is generally supportive and their features are growing over time. ❞
❝ Quick and easy to understand summaries, along with all the necessary links for further analysis. ❞
❝ Knowing when drugs are coming off patent and who to contract with has enabled us to stay ahead fo the curve and align ourselves with the proper vendors. ❞
❝ Drugpatentwatch is an excellent opportunity for LATAM companies to access information on expired and protected patents from the USA and worldwide in a friendly and easy manner. ❞
❝ This is a great service with lots of insightful information. ❞
❝ I like the interface, and how it updates with news ❞
❝ Found the results very comprehensive. The information told me who potential competitors are and what they are planning based on not only their NDAs and patents, but also references to clinical trials. ❞
Start working smarter.
See how companies in more than 70 countries are using DrugPatentWatch.
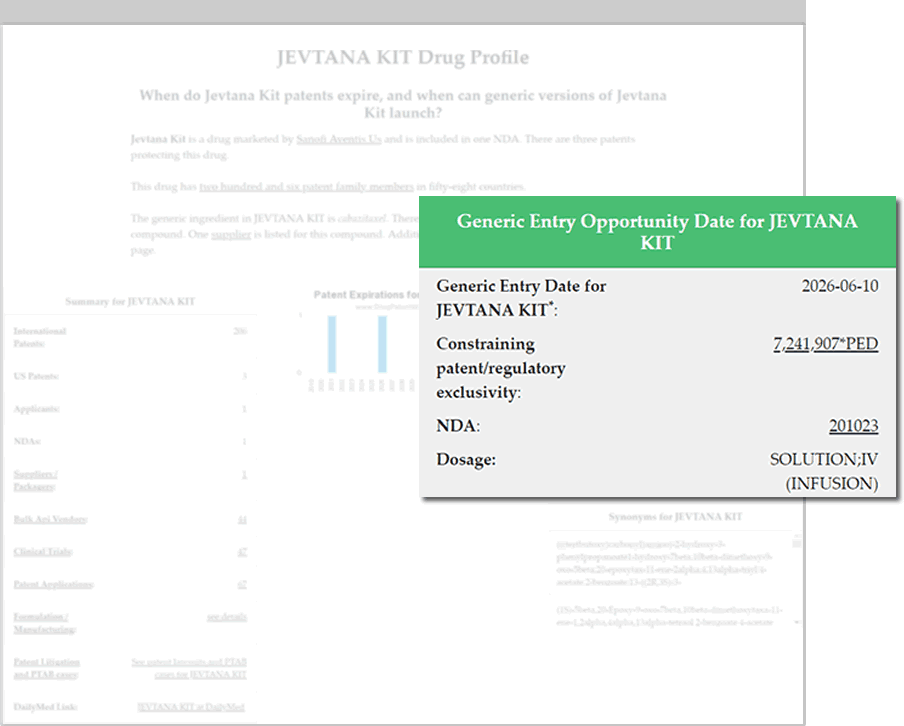
Global Biopharmaceutical Business Intelligence
- Identify and evaluate commercial opportunities
- Branded and generic drug pipeline forecasting
- Anticipate future revenue events
- Identify API and finished drug product suppliers
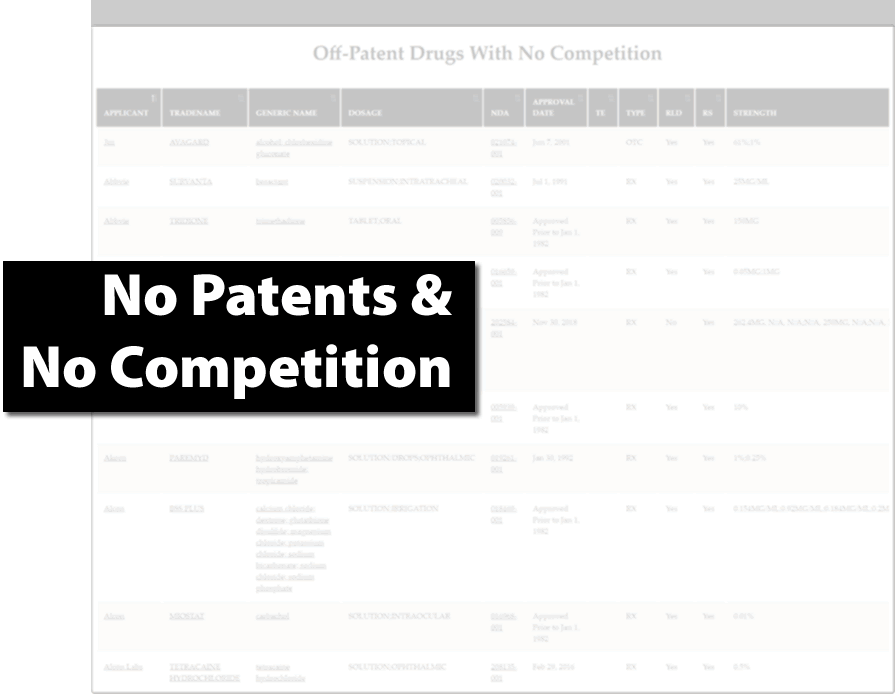
Find Generic Drug Entry Opportunities
- Inform portfolio management decisions
- Sector landscaping and due diligence
- Track investigational drugs and explore new indications for existing drugs
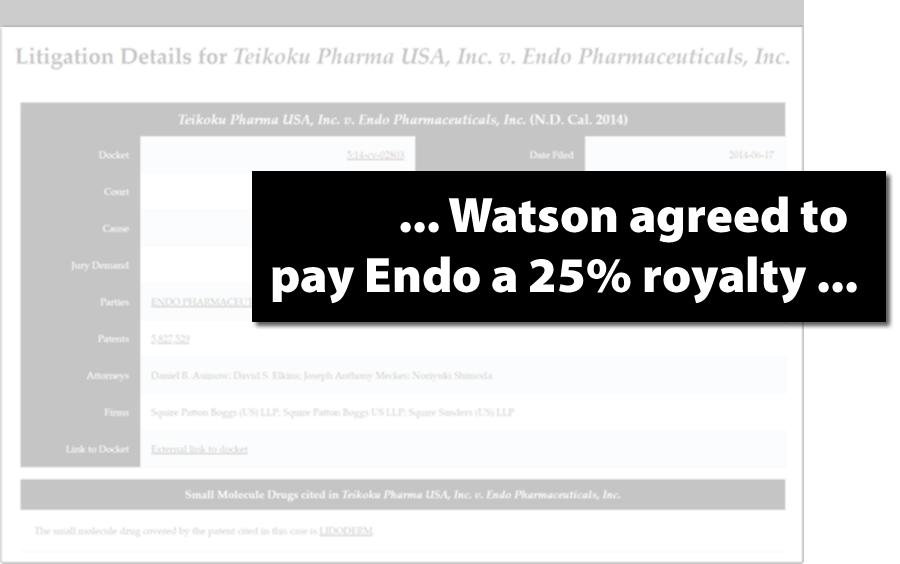
Get Confidential Royalty and Settlement Terms
- Study failed patent challenges to develop a better strategy
- Collect competitive intelligence by examining contractual disputes
- Track litigation to anticipate early generic entry
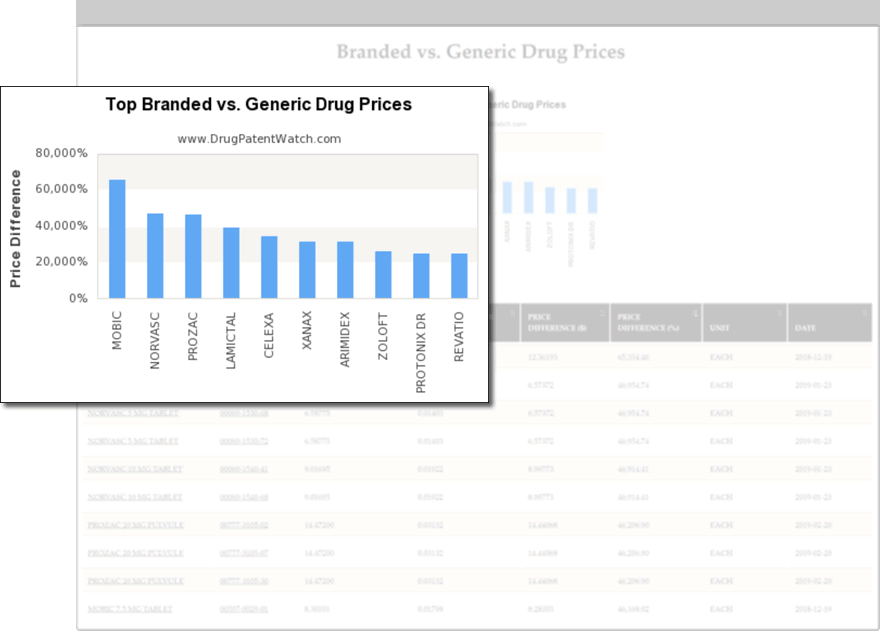
Find and Evaluate Business Opportunities
- Assess levels of generic competition
- Use drug price ranges to evaluate price elasticity
- Determine optimal prices before launch
Automated Reports & Custom Dashboards
- Do more with less staff
- Take the load off your team
- Automate processes and focus on growing your business
Monitor biosimilar and 505(b)(2) activity
- Anticipate 505(b)(2) and biosimilar approvals
- Track OTC-switches, new formulations, biosimilars, and other drug improvements
- Strengthen new formulation patents by studying prior claims and litigation