VEKLURY Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Veklury, and what generic alternatives are available?
Veklury is a drug marketed by Gilead Sciences Inc and is included in one NDA. There are sixteen patents protecting this drug and one Paragraph IV challenge.
This drug has three hundred and forty-three patent family members in forty-nine countries.
The generic ingredient in VEKLURY is remdesivir. One supplier is listed for this compound. Additional details are available on the remdesivir profile page.
DrugPatentWatch® Generic Entry Outlook for Veklury
Veklury was eligible for patent challenges on October 22, 2024.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be April 29, 2036. This may change due to patent challenges or generic licensing.
There have been four patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for VEKLURY?
- What are the global sales for VEKLURY?
- What is Average Wholesale Price for VEKLURY?
Summary for VEKLURY
| International Patents: | 343 |
| US Patents: | 16 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 34 |
| Clinical Trials: | 8 |
| Patent Applications: | 3,446 |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for VEKLURY |
| What excipients (inactive ingredients) are in VEKLURY? | VEKLURY excipients list |
| DailyMed Link: | VEKLURY at DailyMed |
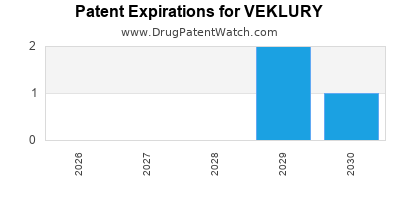
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for VEKLURY
Generic Entry Date for VEKLURY*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
POWDER;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for VEKLURY
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Chiang Mai University | PHASE2 |
| PENTA Foundation | PHASE2 |
| AMS-PHPT Research Collaboration | PHASE2 |
Pharmacology for VEKLURY
Paragraph IV (Patent) Challenges for VEKLURY
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| VEKLURY | Powder for Injection | remdesivir | 100 mg/vial | 214787 | 1 | 2025-04-22 |
US Patents and Regulatory Information for VEKLURY
VEKLURY is protected by sixteen US patents and eight FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of VEKLURY is ⤷ Get Started Free.
This potential generic entry date is based on patent 9,724,360.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gilead Sciences Inc | VEKLURY | remdesivir | SOLUTION;INTRAVENOUS | 214787-002 | Oct 22, 2020 | DISCN | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Gilead Sciences Inc | VEKLURY | remdesivir | SOLUTION;INTRAVENOUS | 214787-002 | Oct 22, 2020 | DISCN | Yes | No | 9,949,994*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Gilead Sciences Inc | VEKLURY | remdesivir | SOLUTION;INTRAVENOUS | 214787-002 | Oct 22, 2020 | DISCN | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Gilead Sciences Inc | VEKLURY | remdesivir | SOLUTION;INTRAVENOUS | 214787-002 | Oct 22, 2020 | DISCN | Yes | No | 11,007,208*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Gilead Sciences Inc | VEKLURY | remdesivir | SOLUTION;INTRAVENOUS | 214787-002 | Oct 22, 2020 | DISCN | Yes | No | 8,318,682*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Gilead Sciences Inc | VEKLURY | remdesivir | SOLUTION;INTRAVENOUS | 214787-002 | Oct 22, 2020 | DISCN | Yes | No | 11,382,926*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Gilead Sciences Inc | VEKLURY | remdesivir | POWDER;INTRAVENOUS | 214787-001 | Oct 22, 2020 | RX | Yes | Yes | 11,492,353*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for VEKLURY
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Gilead Sciences Ireland UC | Veklury | remdesivir | EMEA/H/C/005622Veklury is indicated for the treatment of coronavirus disease 2019 (COVID 19) in:adults and paediatric patients (at least 4 weeks of age and weighing at least 3 kg) with pneumonia requiring supplemental oxygen (low- or high-flow oxygen or other non-invasive ventilation at start of treatment)adults and paediatric patients (weighing at least 40 kg) who do not require supplemental oxygen and who are at increased risk of progressing to severe COVID-19 | Authorised | no | no | no | 2020-07-03 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for VEKLURY
When does loss-of-exclusivity occur for VEKLURY?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2467
Patent: MÉTODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Patent: 2468
Patent: MÉTODOS PARA LA PREPARACIÓN DE RIBÓSIDOS
Estimated Expiration: ⤷ Get Started Free
Patent: 9850
Patent: MÉTODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 15339222
Patent: Methods for the preparation of ribosides
Estimated Expiration: ⤷ Get Started Free
Patent: 15339223
Patent: Methods for treating Filoviridae virus infections
Estimated Expiration: ⤷ Get Started Free
Patent: 18253483
Patent: Methods for treating Filoviridae virus infections
Estimated Expiration: ⤷ Get Started Free
Patent: 19201232
Patent: Methods for the preparation of ribosides
Estimated Expiration: ⤷ Get Started Free
Patent: 20203892
Patent: Methods for treating Filoviridae virus infections
Estimated Expiration: ⤷ Get Started Free
Patent: 21201474
Patent: Methods for the preparation of ribosides
Estimated Expiration: ⤷ Get Started Free
Patent: 22283772
Patent: Methods for treating Filoviridae virus infections
Estimated Expiration: ⤷ Get Started Free
Patent: 23202679
Patent: Methods for the preparation of ribosides
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2015027413
Patent: métodos para tratar infecções pelo vírus filoviridae
Estimated Expiration: ⤷ Get Started Free
Patent: 2017007636
Patent: métodos para a preparação de ribosídeos
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 63832
Patent: METHODES POUR LE TRAITEMENT D'INFECTIONS VIRALES A FILOVIRIDAE (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 63907
Patent: PROCEDES DE PREPARATION DE RIBOSIDES (METHODS FOR THE PREPARATION OF RIBOSIDES)
Estimated Expiration: ⤷ Get Started Free
Patent: 84285
Patent: PROCEDES DE PREPARATION DE RIBOSIDES (METHODS FOR THE PREPARATION OF RIBOSIDES)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 17001040
Patent: Métodos para tratar infecciones por el virus filoviridae
Estimated Expiration: ⤷ Get Started Free
Patent: 17002693
Patent: Métodos para tratar infecciones por el virus filoviridae. divisional de solicitud n° 1040-2017.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 7073005
Patent: 治疗丝状病毒科病毒感染的方法 (Methods for treating filoviridae virus infections)
Estimated Expiration: ⤷ Get Started Free
Patent: 7074902
Patent: 制备核糖核苷的方法 (Methods for the preparation of ribosides)
Estimated Expiration: ⤷ Get Started Free
Patent: 3549120
Patent: 制备核糖核苷的方法 (Method for preparing ribonucleoside)
Estimated Expiration: ⤷ Get Started Free
Patent: 3620992
Patent: 治疗丝状病毒科病毒感染的方法 (Method for treating filoviridae virus infection)
Estimated Expiration: ⤷ Get Started Free
Patent: 4191438
Patent: 治疗丝状病毒科病毒感染的方法 (Methods of treating filoviridae virus infections)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 17003960
Patent: Métodos para tratar infecciones por el virus filoviridae
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 170165
Patent: MÉTODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Patent: 170483
Patent: MÉTODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE (Divisional 2017-165)
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0181130
Estimated Expiration: ⤷ Get Started Free
Patent: 0200518
Estimated Expiration: ⤷ Get Started Free
Cuba
Patent: 170056
Patent: MÉTODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Patent: 170145
Patent: MÉTODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 20893
Estimated Expiration: ⤷ Get Started Free
Patent: 22946
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 12174
Estimated Expiration: ⤷ Get Started Free
Patent: 66295
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 017000103
Patent: MÉTODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 17025261
Patent: MÉTODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Patent: 17072474
Patent: MÉTODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
El Salvador
Patent: 17005424
Patent: MÉTODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Patent: 17005561
Patent: METODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 2239
Patent: СПОСОБЫ ЛЕЧЕНИЯ ВИРУСНЫХ ИНФЕКЦИЙ FILOVIRIDAE (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 9561
Patent: СОЕДИНЕНИЯ ДЛЯ ЛЕЧЕНИЯ ВИРУСНЫХ ИНФЕКЦИЙ FILOVIRIDAE (COMPOUNDS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 1790597
Patent: СПОСОБЫ ЛЕЧЕНИЯ ВИРУСНЫХ ИНФЕКЦИЙ FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Patent: 1790630
Patent: СПОСОБЫ ПОЛУЧЕНИЯ РИБОЗИДОВ
Estimated Expiration: ⤷ Get Started Free
Patent: 1990021
Patent: СПОСОБЫ ЛЕЧЕНИЯ ВИРУСНЫХ ИНФЕКЦИЙ FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 12174
Patent: MÉTHODES POUR LE TRAITEMENT D'INFECTIONS VIRALES À FILOVIRIDAE (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 12175
Patent: PROCÉDÉS DE PRÉPARATION DE RIBOSIDES (METHODS FOR THE PREPARATION OF RIBOSIDES)
Estimated Expiration: ⤷ Get Started Free
Patent: 66295
Patent: PROCÉDÉS DE TRAITEMENT D'INFECTIONS PAR DES VIRUS FILOVIRIDÉS (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 95844
Patent: PROCÉDÉS DE TRAITEMENT D'INFECTIONS PAR DES VIRUS NIPAH (METHODS FOR TREATING NIPAH VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 36099
Patent: MÉTHODES POUR LA PRÉPARATION DE RIBOSIDES (METHODS FOR THE PREPARATION OF RIBOSIDES)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 58795
Patent: 絲狀病毒科病毒感染的治療方法 (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 39231
Estimated Expiration: ⤷ Get Started Free
Patent: 49192
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1707
Patent: שיטות לטיפול בזיהומי וירוס פילווירידאה (Methods for treating filoviridae virus infections)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 20484
Estimated Expiration: ⤷ Get Started Free
Patent: 87547
Estimated Expiration: ⤷ Get Started Free
Patent: 71424
Estimated Expiration: ⤷ Get Started Free
Patent: 57294
Estimated Expiration: ⤷ Get Started Free
Patent: 58428
Estimated Expiration: ⤷ Get Started Free
Patent: 17186358
Patent: フィロウイルス科ウイルス感染症を処置するための方法 (METHOD FOR TREATING FILOVIRIDAE VIRUS INFECTION)
Estimated Expiration: ⤷ Get Started Free
Patent: 17533903
Patent: リボシドの調製のための方法
Estimated Expiration: ⤷ Get Started Free
Patent: 17534614
Patent: フィロウイルス科ウイルス感染症を処置するための方法
Estimated Expiration: ⤷ Get Started Free
Patent: 18172424
Patent: リボシドの調製のための方法 (METHODS FOR PREPARATION OF RIBOSIDES)
Estimated Expiration: ⤷ Get Started Free
Patent: 19048901
Patent: リボシドの調製のための方法 (METHODS FOR PREPARATION OF RIBOSIDES)
Estimated Expiration: ⤷ Get Started Free
Patent: 20090536
Patent: フィロウイルス科ウイルス感染症を処置するための方法 (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 20097635
Patent: リボシドの調製のための方法 (METHODS FOR PREPARING RIBOSIDES)
Estimated Expiration: ⤷ Get Started Free
Patent: 22065066
Patent: フィロウイルス科ウイルス感染症を処置するための方法
Estimated Expiration: ⤷ Get Started Free
Patent: 22068297
Patent: リボシドの調製のための方法
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 12174
Estimated Expiration: ⤷ Get Started Free
Patent: 66295
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 5823
Patent: METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 17005250
Patent: METODOS PARA TRATAR INFECCIONES POR EL VIRUS DE FILOVIRIDAE. (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 17005252
Patent: METODOS PARA LA PREPARACION DE RIBOSIDOS. (METHODS FOR THE PREPARATION OF RIBOSIDES.)
Estimated Expiration: ⤷ Get Started Free
Patent: 20012560
Patent: METODOS PARA TRATAR INFECCIONES POR EL VIRUS DE FILOVIRIDAE. (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS.)
Estimated Expiration: ⤷ Get Started Free
Moldova, Republic of
Patent: 170046
Patent: Metode pentru tratamentul infecţiilor virale cu Filoviridae (Methods for treating Filoviridae virus infections)
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 070
Patent: POSTUPCI LIJEČENJA VIRUSNIH FILOVIRIDAE INFEKCIJA (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 867
Patent: MÉTHODES POUR LE TRAITEMENT D'INFECTIONS VIRALES À FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Patent: 872
Patent: MÉTHODES POUR LA PRÉPARATION DE RIBOSIDES
Estimated Expiration: ⤷ Get Started Free
Patent: 201
Patent: PROCÉDÉS DE TRAITEMENT D'INFECTIONS PAR DES VIRUS FILOVIRIDÉS
Estimated Expiration: ⤷ Get Started Free
Patent: 506
Patent: PROCÉDÉS DE TRAITEMENT D'INFECTIONS PAR DES VIRUS NIPAH
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 0803
Patent: Methods for the preparation of ribosides
Estimated Expiration: ⤷ Get Started Free
Patent: 0809
Patent: Methods for treating filoviridae virus infections
Estimated Expiration: ⤷ Get Started Free
Patent: 5328
Patent: Methods for treating filoviridae virus infections
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 171439
Patent: METODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Patent: 180202
Patent: METODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 017500631
Patent: METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 020551055
Patent: METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 12174
Estimated Expiration: ⤷ Get Started Free
Patent: 12175
Estimated Expiration: ⤷ Get Started Free
Patent: 66295
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 12174
Estimated Expiration: ⤷ Get Started Free
Patent: 12175
Estimated Expiration: ⤷ Get Started Free
Patent: 66295
Estimated Expiration: ⤷ Get Started Free
Saudi Arabia
Patent: 7381419
Patent: طرق لعلاج حالات الإصابة بعدوى فيروس من عائلة الفيروسات الخيطية (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 425
Patent: POSTUPCI LEČENJA VIRUSNIH FILOVIRIDAE INFEKCIJA (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 202008772U
Patent: METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS
Estimated Expiration: ⤷ Get Started Free
Patent: 201702903T
Patent: METHODS FOR THE PREPARATION OF RIBOSIDES
Estimated Expiration: ⤷ Get Started Free
Patent: 201702904R
Patent: METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 12174
Estimated Expiration: ⤷ Get Started Free
Patent: 12175
Estimated Expiration: ⤷ Get Started Free
Patent: 66295
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1800414
Patent: METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1822348
Estimated Expiration: ⤷ Get Started Free
Patent: 2337664
Estimated Expiration: ⤷ Get Started Free
Patent: 2453808
Estimated Expiration: ⤷ Get Started Free
Patent: 170066665
Patent: 필로비리다에 바이러스 감염을 치료하는 방법 (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 170067898
Patent: 필로비리다에 바이러스 감염을 치료하는 방법 (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 170077167
Patent: 리보시드의 제조 방법 (METHODS FOR THE PREPARATION OF RIBOSIDES)
Estimated Expiration: ⤷ Get Started Free
Patent: 210152015
Patent: 필로비리다에 바이러스 감염을 치료하는 방법 (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Patent: 220140656
Patent: 필로비리다에 바이러스 감염을 치료하는 방법 (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 74806
Estimated Expiration: ⤷ Get Started Free
Patent: 85034
Estimated Expiration: ⤷ Get Started Free
Patent: 04298
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 87432
Estimated Expiration: ⤷ Get Started Free
Patent: 98444
Estimated Expiration: ⤷ Get Started Free
Patent: 40546
Estimated Expiration: ⤷ Get Started Free
Patent: 67201
Estimated Expiration: ⤷ Get Started Free
Patent: 1629076
Patent: Methods for treating filoviridae virus infections
Estimated Expiration: ⤷ Get Started Free
Patent: 1630925
Patent: Methods for the preparation of ribosides
Estimated Expiration: ⤷ Get Started Free
Patent: 2039526
Patent: Methods for treating Filoviridae virus infections
Estimated Expiration: ⤷ Get Started Free
Patent: 2115098
Patent: Methods for the preparation of ribosides
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1809518
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 1485
Patent: СПОСОБИ ЛІКУВАННЯ ВІРУСНИХ ІНФЕКЦІЙ FILOVIRIDAE (METHODS FOR TREATING FILOVIRIDAE VIRUS INFECTIONS)
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 376
Patent: MÉTODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Patent: 464
Patent: MÉTODOS PARA TRATAR INFECCIONES POR EL VIRUS FILOVIRIDAE
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering VEKLURY around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Hong Kong | 1152709 | ' - 用於抗病毒治療的 '替代咔核苷類似物 (-SUBSTITUTED CARBA-NUCLEOSIDE ANALOGS FOR ANTIVIRAL TREATMENT) | ⤷ Get Started Free |
| World Intellectual Property Organization (WIPO) | 2019014247 | ⤷ Get Started Free | |
| Lithuania | PA2020539 | ⤷ Get Started Free | |
| Hungary | E049192 | ⤷ Get Started Free | |
| Denmark | 3366295 | ⤷ Get Started Free | |
| Japan | 2023528810 | レムデシビル治療方法 | ⤷ Get Started Free |
| Portugal | 2937350 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VEKLURY
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2937350 | LUC00193 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: REMDESIVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; AUTHORISATION NUMBER AND DATE: EU/1/20/1459 20200703 |
| 2595980 | 53/2020 | Austria | ⤷ Get Started Free | PRODUCT NAME: REMDESIVIR ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ ODER ESTER DAVON; REGISTRATION NO/DATE: EU/1/20/1459 (MITTEILUNG) 20200703 |
| 2595980 | 122020000084 | Germany | ⤷ Get Started Free | PRODUCT NAME: REMDESIVIR ODER PHARMAZEUTISCH AKZEPTABLES SALZ ODER PHARMAZEUTISCH AKZEPTABLER ESTER DAVON; REGISTRATION NO/DATE: EU/1/20/1459 20200703 |
| 2595980 | 20C1065 | France | ⤷ Get Started Free | PRODUCT NAME: REMDESIVIR OU UN SEL OU UN ESTER PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; REGISTRATION NO/DATE: EU/1/20/1459 20200703 |
| 2937350 | CA 2020 00060 | Denmark | ⤷ Get Started Free | PRODUCT NAME: REMDESIVIR ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/20/1459 20200703 |
| 2595980 | 2020/061 | Ireland | ⤷ Get Started Free | PRODUCT NAME: REMDESIVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT OR ESTER THEREOF; REGISTRATION NO/DATE: EU/1/20/1459 20200703 |
| 2937350 | PA2020539,C2937350 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: REMDESIVIRAS ARBA JO FARMACINIU POZIURIU PRIIMTINA DRUSKA; REGISTRATION NO/DATE: EU/1/20/1459 20200703 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for VEKLURY (Remdesivir)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
