GEMTESA Drug Patent Profile
✉ Email this page to a colleague
When do Gemtesa patents expire, and when can generic versions of Gemtesa launch?
Gemtesa is a drug marketed by Sumitomo Pharma Am and is included in one NDA. There are five patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and nineteen patent family members in forty-eight countries.
The generic ingredient in GEMTESA is vibegron. One supplier is listed for this compound. Additional details are available on the vibegron profile page.
DrugPatentWatch® Generic Entry Outlook for Gemtesa
Gemtesa was eligible for patent challenges on December 23, 2024.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be April 2, 2029. This may change due to patent challenges or generic licensing.
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for GEMTESA?
- What are the global sales for GEMTESA?
- What is Average Wholesale Price for GEMTESA?
Summary for GEMTESA
| International Patents: | 119 |
| US Patents: | 5 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 27 |
| Clinical Trials: | 3 |
| Patent Applications: | 139 |
| Drug Prices: | Drug price information for GEMTESA |
| What excipients (inactive ingredients) are in GEMTESA? | GEMTESA excipients list |
| DailyMed Link: | GEMTESA at DailyMed |
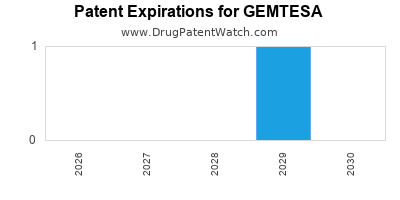
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for GEMTESA
Generic Entry Date for GEMTESA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for GEMTESA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Wake Forest University Health Sciences | PHASE3 |
| University of Missouri-Columbia | EARLY_PHASE1 |
| Urovant Sciences GmbH | Phase 4 |
Pharmacology for GEMTESA
| Drug Class | beta3-Adrenergic Agonist |
| Mechanism of Action | Adrenergic beta3-Agonists |
US Patents and Regulatory Information for GEMTESA
GEMTESA is protected by five US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of GEMTESA is ⤷ Get Started Free.
This potential generic entry date is based on patent 8,653,260.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
International Patents for GEMTESA
When does loss-of-exclusivity occur for GEMTESA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2043
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 09231714
Estimated Expiration: ⤷ Get Started Free
Austria
Patent: 35521
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0909768
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 19876
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 09000815
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2056917
Estimated Expiration: ⤷ Get Started Free
Patent: 2391255
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 31440
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 751
Estimated Expiration: ⤷ Get Started Free
Patent: 120282
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0120129
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 12552
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 76756
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 010000294
Estimated Expiration: ⤷ Get Started Free
Patent: 013000267
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 10010518
Estimated Expiration: ⤷ Get Started Free
El Salvador
Patent: 10003687
Patent: HIDROXIMETIL PIRROLIDINAS COMO AGONISTAS DEL RECEPTOR ADRENERGICO BETA 3
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 0135
Estimated Expiration: ⤷ Get Started Free
Patent: 1071169
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 76756
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 0240046
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1051
Estimated Expiration: ⤷ Get Started Free
Georgia, Republic of
Patent: 0125666
Estimated Expiration: ⤷ Get Started Free
Honduras
Patent: 10002030
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 47099
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 8215
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 83867
Estimated Expiration: ⤷ Get Started Free
Patent: 83870
Estimated Expiration: ⤷ Get Started Free
Patent: 32846
Estimated Expiration: ⤷ Get Started Free
Patent: 11201897
Estimated Expiration: ⤷ Get Started Free
Patent: 11510023
Estimated Expiration: ⤷ Get Started Free
Patent: 12020961
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 10010929
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 988
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 257
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 1305
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 8266
Estimated Expiration: ⤷ Get Started Free
Nicaragua
Patent: 1000164
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 24055
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 091825
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 76756
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 76756
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 175
Patent: HIDROKSIMETIL PIROLIDINI KAO AGONISTI BETA 3 ADRENERGIČKOG RECEPTORA (HYDROXYMETHYL PYRROLIDINES AS BETA 3 ADRENERGIC RECEPTOR AGONISTS)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 8883
Patent: HYDROXYMETHYL PYRROLIDINES AS BETA 3 ADRENERGIC RECEPTOR AGONISTS
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 76756
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1006720
Patent: HYDROXYMETHYL PYRROLIDINES AS BETA 3 ADRENERGIC RECEPTOR AGONISTS
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1288798
Estimated Expiration: ⤷ Get Started Free
Patent: 1331771
Estimated Expiration: ⤷ Get Started Free
Patent: 100126860
Estimated Expiration: ⤷ Get Started Free
Patent: 120104257
Estimated Expiration: ⤷ Get Started Free
Patent: 120118086
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 76278
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 78098
Estimated Expiration: ⤷ Get Started Free
Patent: 0944521
Patent: Hydroxymethyl pyrrolidines as beta 3 adrenergic receptor agonists
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 10000447
Patent: HYDROXYMETHYL PYRROLIDINES AS BETA 3 ADRENERGIC RECEPTOR AGONISTS
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 1367
Patent: ГІДРОКСИМЕТИЛПІРОЛІДИНИ ЯК АГОНІСТИ АДРЕНЕРГІЧНИХ РЕЦЕПТОРІВ β3[Normal;heading 1;heading 2;heading 3;ГИДРОКСИМЕТИЛПИРРОЛИДИНЫ КАК АГОНИСТЫ АДРЕНЕРГИЧЕСКИХ РЕЦЕПТОРОВ β3 (Normal;heading 1;heading 2;heading 3;HYDROXYMETHYL PYRROLIDINES AS BETA 3 ADRENERGIC RECEPTOR AGONISTS)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering GEMTESA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20250099259 | 과민성 방광의 치료를 위한 비베그론의 용도 (USE OF VIBEGRON TO TREAT OVERACTIVE BLADDER) | ⤷ Get Started Free |
| South Korea | 20200012949 | 과민성 방광의 치료를 위한 비베그론의 용도 | ⤷ Get Started Free |
| San Marino | T201900508 | ⤷ Get Started Free | |
| Ecuador | SP10010518 | ⤷ Get Started Free | |
| Japan | 7682605 | ⤷ Get Started Free | |
| Peru | 20091825 | ⤷ Get Started Free | |
| Montenegro | 03506 | POSTUPAK ZA PRIPREMU BETA 3 AGONISTA I MEĐUPROIZVODA (PROCESS FOR PREPARING BETA 3 AGONISTS AND INTERMEDIATES) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for GEMTESA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2276756 | 2490045-8 | Sweden | ⤷ Get Started Free | PRODUCT NAME: VIBEGRON OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REG. NO/DATE: EU/1/24/1822 20240628 |
| 2276756 | CR 2024 00054 | Denmark | ⤷ Get Started Free | PRODUCT NAME: VIBEGRON OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REG. NO/DATE: EU/1/24/1822 20240628 |
| 2276756 | 301305 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: VIBEGRON OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/24/1822 20240628 |
| 2276756 | 49/2024 | Austria | ⤷ Get Started Free | PRODUCT NAME: VIBEGRON ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON; REGISTRATION NO/DATE: EU/1/24/1822 (MITTEILUNG) 20240628 |
| 2276756 | 122024000082 | Germany | ⤷ Get Started Free | PRODUCT NAME: VIBEGRON ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON; REGISTRATION NO/DATE: EU/1/24/1822 20240627 |
| 2276756 | 2024C/551 | Belgium | ⤷ Get Started Free | PRODUCT NAME: VIBEGRON OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT HIERVAN; AUTHORISATION NUMBER AND DATE: EU/1/24/1822 20240628 |
| 2276756 | C20240046 | Finland | ⤷ Get Started Free | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for GEMTESA (Vibegron)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
