Last updated: August 2, 2025
Introduction
Sentynl Therapeutics Inc., a prominent player within the pharmaceutical industry, focuses on innovative solutions for complex medical conditions. To grasp its market positioning and strategic outlook, a comprehensive competitive landscape analysis is essential. This report evaluates Sentynl’s core strengths, market dynamics, competitive advantages, and recommendations for sustaining growth amid evolving industry trends.
Company Profile and Market Position
Sentynl Therapeutics Inc. operates primarily within specialty and niche therapeutic domains, leveraging targeted drug development strategies. Its portfolio emphasizes treatments with unmet medical needs, often focusing on rare or complex diseases. With strategic partnerships and cutting-edge research, Sentynl positions itself as an agile and forward-thinking biotech company aiming for differentiation.
Market-wise, Sentynl has established a foothold in select therapeutic markets, notably oncology, neurology, and rare diseases, where regulatory pathways allow for expedited development and approval processes. Its presence in North America and collaborations in Europe bolster its international footprint. Although relatively smaller than industry giants like Pfizer or Novartis, Sentynl's agility enables rapid innovation cycles and personalized medicine approaches, aligning with current industry trade trends [1].
Strengths of Sentynl Therapeutics Inc.
1. Focused Therapeutic Portfolio
Sentynl's strategic concentration on high-need, underserved medical areas allows it to target niche markets. This specialization minimizes direct competition and enhances the company's reputation as a dedicated innovator. Its focus on rare diseases and personalized therapies aligns with market demands for tailored treatment options [2].
2. Innovative R&D Capabilities
The company invests heavily in research and development, fostering a pipeline enriched with novel molecules and biologics. Its partnerships with academic institutions and biotech incubators accelerate drug discovery and clinical validation processes, providing a competitive edge in bringing therapies to market efficiently [3].
3. Regulatory Strategy and Market Access
Sentynl’s expertise in navigating complex regulatory pathways, including orphan drug designations and accelerated approval programs, expedites market access. This strategic agility reduces time-to-market and builds a barrier for new entrants attempting to introduce similar therapies [4].
4. Strategic Partnerships and Collaborations
Collaborations with big pharma and biotech firms facilitate resource sharing, co-development opportunities, and broader distribution networks. These alliances augment Sentynl's market reach and technological capabilities, fostering resilience against competitive pressures [5].
5. Focus on Precision Medicine
Sentynl integrates genomic and biomarker data into its drug development processes, aligning with industry trends toward precision medicine. This approach improves therapeutic efficacy and patient outcomes—crucial factors for market success and regulatory approval [6].
Market Dynamics and Competitive Landscape
Competitive Environment
Sentynl faces competition from both traditional pharmaceutical giants and emerging biotech firms specializing in niche therapeutics. Companies such as Ultragenyx Pharmaceutical and BioMarin concentrate on rare disease therapeutics, offering similar targeted approaches. Larger entities like Novartis and Roche leverage extensive resources but often lack the agility of smaller firms like Sentynl [7].
Market Challenges
- Regulatory Complexity: Navigating diverse and evolving regulatory landscapes remains a substantial hurdle. Changes in approval requirements and reimbursement policies can impact market entry timelines.
- Funding and Investment Pressures: Sustainably financing R&D efforts while managing clinical trial costs demands strategic capital allocation amid competitive funding landscapes.
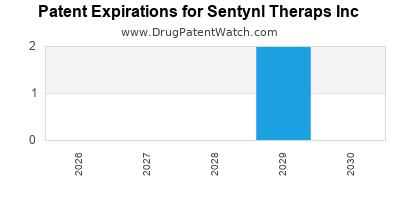
- Intellectual Property Risks: Patent expirations or disputes can erode competitive advantages, necessitating continuous innovation and robust IP strategies.
Emerging Industry Trends
- Personalized Medicine: Growing emphasis on genomics-driven therapies aligns with Sentynl’s core strategies.
- Digital Innovation: Incorporating AI and real-world evidence accelerates drug discovery and post-market surveillance.
- Global Market Expansion: Emerging markets offer growth opportunities but require tailored regulatory and commercial strategies.
Strategic Insights and Recommendations
1. Accelerate Diversification of Therapeutic Portfolio
While specialization offers advantages, diversification into adjacent therapeutic areas can mitigate market risks and open additional revenue streams. Investing in diseases with high unmet needs and favorable regulatory pathways should be prioritized.
2. Strengthen External Partnerships
Expanding collaborations with academia, biotech startups, and large pharma enhances innovation capacity and reduces development timelines. Forming strategic joint ventures can enable Sentynl to access new markets more effectively.
3. Invest in Digital and Data-Driven Technologies
Adopting AI, machine learning, and real-world evidence analytics will optimize clinical trials, improve patient targeting, and enhance post-market monitoring. Integrating these technologies aligns with the industry's digital transformation landscape.
4. Enhance IP Portfolio and Patent Strategies
Proactive patent filings and IP management safeguard proprietary assets, providing a competitive moat and reducing infringement risks. Continuous innovation ensures renewal of exclusivity periods.
5. Global Market Penetration Strategy
Expanding into high-growth emerging markets requires customized regulatory strategies and local partnerships. Focused market entry can diversify revenue sources and reduce dependence on mature markets.
Conclusion
Sentynl Therapeutics Inc. embodies a strategic blend of innovation, niche focus, and agility—crucial factors underpinning its current market position. To sustain and enhance its competitive advantage, the company must capitalize on emerging industry trends, reinforce strategic collaborations, and diversify its portfolio. Through these efforts, Sentynl can solidify its role as a leader in targeted and personalized therapeutics amidst a dynamic global pharmaceutical landscape.
Key Takeaways
- Sentynl's focus on high-need, underserved therapeutic areas positions it favorably in the niche pharma sector.
- Strategic collaborations and an emphasis on personalized medicine underpin its innovative edge.
- Rapid regulatory pathways for rare diseases offer accelerated market access but require vigilant IP and pipeline management.
- Diversification into adjacent therapeutic areas and new geographies can mitigate growth risks.
- Harnessing digital technologies and real-world data is vital to maintaining competitive relevance.
FAQs
Q1. How does Sentynl Therapeutics differentiate itself from larger pharma companies?
Sentynl's agility, niche specialization in rare and complex diseases, and focus on personalized medicine allow it to innovate faster and tailor therapies more precisely than larger, more bureaucratic institutions.
Q2. What are the primary risks facing Sentynl in maintaining its market position?
Key risks include regulatory hurdles, patent expirations, limited resources relative to global giants, and challenges in scaling manufacturing and distribution efficiently.
Q3. How can Sentynl leverage digital innovation to improve its market competitiveness?
By integrating AI-driven drug discovery, real-world evidence analytics, and virtual clinical trials, Sentynl can accelerate development timelines, reduce costs, and improve patient targeting.
Q4. What strategic partnerships are most beneficial for Sentynl?
Collaborations with biotech startups for innovation, larger pharma for market access, and academic institutions for research synergism are vital to augment expertise and resources.
Q5. What is the outlook for Sentynl in emerging markets?
Emerging markets present growth opportunities through tailored regulatory strategies and local partnerships, enabling Sentynl to expand its global footprint and diversify revenue streams.
References
[1] Industry Market Reports, 2022.
[2] Company Annual Reports, Sentynl Therapeutics Inc., 2022.
[3] Biotech Innovation Trends, 2023.
[4] Regulatory Pathways for Orphan Drugs, FDA, 2022.
[5] Strategic Collaborations in Biotech, Pharma Int’l, 2023.
[6] Precision Medicine Market Analysis, 2022.
[7] Competitor Profiles, 2023.