VERQUVO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Verquvo, and when can generic versions of Verquvo launch?
Verquvo is a drug marketed by MSD and is included in one NDA. There are six patents protecting this drug and one Paragraph IV challenge.
This drug has two hundred and fourteen patent family members in fifty countries.
The generic ingredient in VERQUVO is vericiguat. One supplier is listed for this compound. Additional details are available on the vericiguat profile page.
DrugPatentWatch® Generic Entry Outlook for Verquvo
Verquvo was eligible for patent challenges on January 19, 2025.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be November 26, 2032. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for VERQUVO?
- What are the global sales for VERQUVO?
- What is Average Wholesale Price for VERQUVO?
Summary for VERQUVO
| International Patents: | 214 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 52 |
| Clinical Trials: | 2 |
| Patent Applications: | 331 |
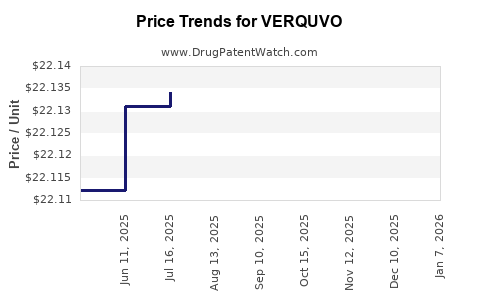
| Drug Prices: | Drug price information for VERQUVO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for VERQUVO |
| What excipients (inactive ingredients) are in VERQUVO? | VERQUVO excipients list |
| DailyMed Link: | VERQUVO at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for VERQUVO
Generic Entry Date for VERQUVO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for VERQUVO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Johns Hopkins University | Phase 2 |
| Merck Sharp & Dohme LLC | Phase 2 |
| Josef Stehlik | Phase 4 |
Pharmacology for VERQUVO
| Drug Class | Soluble Guanylate Cyclase Stimulator |
| Mechanism of Action | Guanylate Cyclase Stimulators |
Paragraph IV (Patent) Challenges for VERQUVO
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| VERQUVO | Tablets | vericiguat | 5 mg and 10 mg | 214377 | 4 | 2025-01-21 |
US Patents and Regulatory Information for VERQUVO
VERQUVO is protected by six US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of VERQUVO is ⤷ Get Started Free.
This potential generic entry date is based on patent 9,604,948.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Msd | VERQUVO | vericiguat | TABLET;ORAL | 214377-001 | Jan 19, 2021 | RX | Yes | No | 9,993,476 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Msd | VERQUVO | vericiguat | TABLET;ORAL | 214377-003 | Jan 19, 2021 | RX | Yes | Yes | 11,439,642 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Msd | VERQUVO | vericiguat | TABLET;ORAL | 214377-001 | Jan 19, 2021 | RX | Yes | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Msd | VERQUVO | vericiguat | TABLET;ORAL | 214377-001 | Jan 19, 2021 | RX | Yes | No | 11,439,642 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Msd | VERQUVO | vericiguat | TABLET;ORAL | 214377-002 | Jan 19, 2021 | RX | Yes | No | 10,736,896 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Msd | VERQUVO | vericiguat | TABLET;ORAL | 214377-003 | Jan 19, 2021 | RX | Yes | Yes | 8,420,656 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Msd | VERQUVO | vericiguat | TABLET;ORAL | 214377-002 | Jan 19, 2021 | RX | Yes | No | 8,921,377 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for VERQUVO
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Bayer AG | Verquvo | vericiguat | EMEA/H/C/005319Treatment of symptomatic chronic heart failure | Authorised | no | no | no | 2021-07-16 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for VERQUVO
When does loss-of-exclusivity occur for VERQUVO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 98
Patent: Method for producing substituted 5-fluoro-1H-pyrazolopyridines
Estimated Expiration: ⤷ Get Started Free
Argentina
Patent: 8983
Patent: PROCEDIMIENTO PARA LA PREPARACION DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Patent: 5027
Patent: PROCEDIMIENTO PARA LA PREPARACIÓN DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 12342547
Patent: Method for producing substituted 5-fluoro-1H-pyrazolopyridines
Estimated Expiration: ⤷ Get Started Free
Patent: 17254916
Patent: Method for producing substituted 5-fluoro-1H-pyrazolopyridines
Estimated Expiration: ⤷ Get Started Free
Patent: 19202123
Patent: Method for producing substituted 5-fluoro-1H-pyrazolopyridines
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2014012414
Patent: processo para a preparação de 5-flúor-1h-pirazolopiridinas substituídas
Estimated Expiration: ⤷ Get Started Free
Patent: 2020001312
Patent: Processo para preparação de 5-flúor-1h-pirazolopiridinas substituídas, e compostos intermediários
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 56706
Patent: PROCEDE DE PRODUCTION DE 5-FLUORO-1H-PYRAZOLOPYRIDINES SUBSTITUEES (METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES)
Estimated Expiration: ⤷ Get Started Free
Patent: 40720
Patent: PROCEDE DE PRODUCTION DE 5-FLUORO-1H-PYRAZOLOPYRIDINES SUBSTITUEES (METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 14001339
Patent: Procedimiento de preparacion de un compuesto intermediario derivado de 5-fluoro-1h-pirazolopiridina; compuestos intermediarios que participan del mismo y formas cristalinas del compuesto final obtenido, el cual es util en el tratamiento de trastornos cardiocirculatorios.
Estimated Expiration: ⤷ Get Started Free
Patent: 16000344
Patent: Procedimiento de preparación de 5-fluoro-1h-pirazolopiridinas sustituidas
Estimated Expiration: ⤷ Get Started Free
China
Patent: 4159898
Patent: METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES
Estimated Expiration: ⤷ Get Started Free
Patent: 5503867
Patent: Method for producing substituted 5-fluoro-lH-pyrazolopyridines
Estimated Expiration: ⤷ Get Started Free
Patent: 6905314
Patent: 用于制备取代的5‑氟‑1H‑吡唑并吡啶类化合物的方法 (Method for producing substituted 5-fluoro-1H-pyrazolopyridines)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 60555
Patent: Procedimiento de preparación de 5-fluoro-1h-pirazolopiridinas sustituidas
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 140237
Patent: PROCEDIMIENTO DE PREPARACIÓN DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Patent: 190057
Patent: PROCEDIMIENTO DE PREPARACIÓN DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS (Divisional 2014-0237) (METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES)
Estimated Expiration: ⤷ Get Started Free
Patent: 210072
Patent: PROCEDIMIENTO DE PREPARACIÓN DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS (METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES)
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0161501
Estimated Expiration: ⤷ Get Started Free
Patent: 0181818
Estimated Expiration: ⤷ Get Started Free
Patent: 0210507
Estimated Expiration: ⤷ Get Started Free
Cuba
Patent: 257
Patent: PROCEDIMIENTO DE PREPARACIÓN DE 5- FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Patent: 354
Patent: PROCEDIMIENTO DE OBTENCIÓN DE DERIVADOS DE TETRAFLUOROPROPILMORFOLINA
Estimated Expiration: ⤷ Get Started Free
Patent: 140055
Patent: PROCEDIMIENTO DE PREPARACIÓN DE 5- FLUORO- 1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Patent: 150123
Patent: DOS FORMAS CRISTALINAS DEL COMPUESTO( 4,6-DIAMINO-2-(5-FLUORO-1-(2-FLUOROBENCIL)-1H-PIRAZOLO?(3,4-b)PIRIDIN-3-IL)PIRIMIDIN-5-IL)CARBAMATO DE METILO
Estimated Expiration: ⤷ Get Started Free
Patent: 150124
Patent: PROCEDIMIENTO DE OBTENCIÓN DE DERIVADOS DE TETRAFLUOROPROPILMORFOLINA
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 24000
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 82914
Estimated Expiration: ⤷ Get Started Free
Patent: 96617
Estimated Expiration: ⤷ Get Started Free
Patent: 21470
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 014000112
Patent: PROCEDIMIENTO DE PREPARACIÓN DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Patent: 017000013
Patent: PROCEDIMIENTO DE PREPARACIÓN DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Patent: 021000179
Patent: PROCEDIMIENTO DE PREPARACIÓN DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 14001627
Patent: PROCEDIMIENTO DE PREPARACIÓN DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 0018
Patent: СПОСОБ ПОЛУЧЕНИЯ ЗАМЕЩЕННОГО 5-ФТОР-1H-ПИРАЗОЛОПИРИДИНА (METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINE)
Estimated Expiration: ⤷ Get Started Free
Patent: 1602
Patent: СПОСОБЫ ПОЛУЧЕНИЯ АЛЬДЕГИДОВ (PROCESSES FOR PRERARING ALDEHYDES)
Estimated Expiration: ⤷ Get Started Free
Patent: 3455
Patent: КРИСТАЛЛИЧЕСКИЕ ФОРМЫ ЗАМЕЩЕННЫХ 5-ФТОР-1H-ПИРАЗОЛОПИРИДИНОВ, СПОСОБЫ ИХ ПОЛУЧЕНИЯ, ИХ ПРИМЕНЕНИЕ ДЛЯ ПОЛУЧЕНИЯ ЛЕКАРСТВЕННОГО СРЕДСТВА, ЛЕКАРСТВЕННОЕ СРЕДСТВО И СПОСОБ ЛЕЧЕНИЯ НА ИХ ОСНОВЕ (CRYSTALLINE FORMS OF SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES, PROCESSES FOR PREPARING SAME, USE THEREOF FOR PRODUCING A MEDICAMENT, MEDICAMENT AND METHOD OF TREATMENT BASED THEREOF)
Estimated Expiration: ⤷ Get Started Free
Patent: 1491028
Patent: СПОСОБ ПОЛУЧЕНИЯ ЗАМЕЩЕННЫХ 5-ФТОР-1Н-ПИРАЗОЛОПИРИДИНОВ
Estimated Expiration: ⤷ Get Started Free
Patent: 1690520
Patent: СПОСОБ ПОЛУЧЕНИЯ ЗАМЕЩЕННЫХ 5-ФТОР-1H-ПИРАЗОЛОПИРИДИНОВ
Estimated Expiration: ⤷ Get Started Free
Patent: 1690521
Patent: СПОСОБ ПОЛУЧЕНИЯ ЗАМЕЩЕННЫХ 5-ФТОР-1H-ПИРАЗОЛОПИРИДИНОВ
Estimated Expiration: ⤷ Get Started Free
Patent: 1892050
Patent: СПОСОБ ПОЛУЧЕНИЯ ЗАМЕЩЕННЫХ 5-ФТОР-1H-ПИРАЗОЛОПИРИДИНОВ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 82914
Patent: PROCÉDÉ DE PRODUCTION DE 5-FLUORO-1H-PYRAZOLOPYRIDINES SUBSTITUÉES (METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES)
Estimated Expiration: ⤷ Get Started Free
Patent: 96617
Patent: PROCÉDÉ DE PRODUCTION DE (Z)-ALPHA-FLUORO-BETA-AMINO-ACRYLALDEHYDES SUBSTITUÉES (METHOD FOR PRODUCING SUBSTITUTED (Z)-ALPHA-FLUORO-BETA-AMINO-ACRYLALDEHYDES)
Estimated Expiration: ⤷ Get Started Free
Patent: 21470
Patent: 5-FLUORO-1H-PYRAZOLOPYRIDINES SUBSTITUÉES SOUS FORME CRISTALLINE (SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES IN CRYSTALLINE FORM)
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 1400101
Patent: PROCEDIMIENTO DE PREPARACIÓN DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 03951
Patent: 用於製備取代的 -氟- -吡唑並吡啶類化合物的方法 (METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES 5--1H-)
Estimated Expiration: ⤷ Get Started Free
Patent: 23613
Patent: 用於製備取代的 -氟- -吡唑並吡啶類化合物的方法 (METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES 5--1H-)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 31029
Estimated Expiration: ⤷ Get Started Free
Patent: 41592
Estimated Expiration: ⤷ Get Started Free
Patent: 53745
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 6265
Patent: שיטה לייצור חומרי ביניים להכנת 5-פלואורו-h1-פיראזולופירידינים ותרכובות שכאלה (Process for preparing intermediates for the preparation of 5-fluoro-1h-pyrazolopyridines and some such compounds)
Estimated Expiration: ⤷ Get Started Free
Patent: 3389
Patent: שיטה לייצור 5-פלואורו-1h-פיראזולופירידינים מותמרים (Method for producing substituted 5-fluoro-1h-pyrazolopyridines)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 89315
Estimated Expiration: ⤷ Get Started Free
Patent: 40391
Estimated Expiration: ⤷ Get Started Free
Patent: 92436
Estimated Expiration: ⤷ Get Started Free
Patent: 15502932
Patent: 置換された5−フルオロ−1H−ピラゾロピリジン類を製造するための方法
Estimated Expiration: ⤷ Get Started Free
Patent: 17031180
Patent: 置換された5−フルオロ−1H−ピラゾロピリジン類を製造するための方法 (PROCESS FOR PREPARING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES)
Estimated Expiration: ⤷ Get Started Free
Patent: 18058860
Patent: 置換された5−フルオロ−1H−ピラゾロピリジン類を製造するための方法 (PROCESS FOR PREPARING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES)
Estimated Expiration: ⤷ Get Started Free
Patent: 18199698
Patent: 置換された5−フルオロ−1H−ピラゾロピリジン類を製造するための方法 (METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLO PYRIDINES)
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 0120351
Patent: عملية لتحضير 5- فلورو-1H- بيرازولوبيريدينات مستبدلة (Process for preparing substituted 5-fluoro-1H-pyrazolopyridines)
Estimated Expiration: ⤷ Get Started Free
Patent: 0210064
Patent: عملية لتحضير 5- فلورو-1H- بيرازولوبيريدينات مستبدلة (Process for preparing substituted 5-fluoro-1H-pyrazolopyridines)
Estimated Expiration: ⤷ Get Started Free
Patent: 0210065
Patent: عملية لتحضير 5- فلورو-1H- بيرازولوبيريدينات مستبدلة (Process for preparing substituted 5-fluoro-1H-pyrazolopyridines)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 82914
Estimated Expiration: ⤷ Get Started Free
Patent: 96617
Estimated Expiration: ⤷ Get Started Free
Patent: 21470
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 8412
Patent: METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES
Estimated Expiration: ⤷ Get Started Free
Patent: 7904
Patent: METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES
Estimated Expiration: ⤷ Get Started Free
Patent: 8086
Patent: METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 7481
Patent: PROCEDIMIENTO DE PREPARACIÓN DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS. (METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES.)
Estimated Expiration: ⤷ Get Started Free
Patent: 14006018
Patent: PROCEDIMIENTO DE PREPARACION DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS. (METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES.)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 718
Patent: Procédé de production de 5-fluoro-1h-pyrazolopyridines substituées
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 4593
Patent: Method for producing substituted 5-fluoro-1h-pyrazolopyridines
Estimated Expiration: ⤷ Get Started Free
Patent: 1592
Patent: Method for producing substituted 5-fluoro-1h-pyrazolopyridines
Estimated Expiration: ⤷ Get Started Free
Patent: 4215
Patent: Method for producing substituted 5-fluoro-1h-pyrazolopyridines
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 142359
Patent: PROCEDIMIENTO DE PREPARACION DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Patent: 190180
Patent: PROCEDIMIENTO DE PREPARACION DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Patent: 190181
Patent: PROCEDIMIENTO DE PREPARACION DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 014501160
Patent: METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H - PYRAZOLOPYRIDINES
Estimated Expiration: ⤷ Get Started Free
Patent: 015501494
Patent: METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIMIDINES
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 82914
Estimated Expiration: ⤷ Get Started Free
Patent: 96617
Estimated Expiration: ⤷ Get Started Free
Patent: 21470
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 82914
Estimated Expiration: ⤷ Get Started Free
Patent: 96617
Estimated Expiration: ⤷ Get Started Free
Patent: 21470
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 387
Patent: POSTUPAK ZA DOBIJANJE SUPSTITUISANIH (Z)-ALFA-FLUORO-BETA-AMINO-AKRILALDEHIDA (METHOD FOR PRODUCING SUBSTITUTED (Z)-ALPHA-FLUORO-BETA-AMINO-ACRYLALDEHYDES)
Estimated Expiration: ⤷ Get Started Free
Patent: 945
Patent: POSTUPAK ZA DOBIJANJE SUPSTITUISANIH 5-FLUORO-1H-PIRAZOLOPIRADINA (METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES)
Estimated Expiration: ⤷ Get Started Free
Patent: 609
Patent: SUPSTITUISANI 5-FLUOR-1H-PIRAZOLOPIRIDIN U KRISTALNOM OBLIKU (SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES IN CRYSTALLINE FORM)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201604192X
Patent: METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES
Estimated Expiration: ⤷ Get Started Free
Patent: 201604196V
Patent: METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES
Estimated Expiration: ⤷ Get Started Free
Patent: 201402111Q
Patent: METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 82914
Estimated Expiration: ⤷ Get Started Free
Patent: 96617
Estimated Expiration: ⤷ Get Started Free
Patent: 21470
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1403613
Patent: METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES
Estimated Expiration: ⤷ Get Started Free
Patent: 1505970
Patent: METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1943788
Estimated Expiration: ⤷ Get Started Free
Patent: 1993022
Estimated Expiration: ⤷ Get Started Free
Patent: 2026059
Estimated Expiration: ⤷ Get Started Free
Patent: 140105483
Patent: METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES
Estimated Expiration: ⤷ Get Started Free
Patent: 170130612
Patent: 치환된 5-플루오로-1H-피라졸로피리딘의 제조 방법 (5--1H- METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES)
Estimated Expiration: ⤷ Get Started Free
Patent: 190018021
Patent: 치환된 5-플루오로-1H-피라졸로피리딘의 제조 방법 (5--1H- METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 03028
Estimated Expiration: ⤷ Get Started Free
Patent: 94158
Estimated Expiration: ⤷ Get Started Free
Patent: 64009
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 97280
Estimated Expiration: ⤷ Get Started Free
Patent: 31123
Estimated Expiration: ⤷ Get Started Free
Patent: 59016
Estimated Expiration: ⤷ Get Started Free
Patent: 65198
Estimated Expiration: ⤷ Get Started Free
Patent: 1329076
Patent: Process for preparing substituted 5-fluoro-1H-pyrazolopyridines
Estimated Expiration: ⤷ Get Started Free
Patent: 1708219
Patent: Process for preparing substituted 5-fluoro-1H-pyrazolopyridines
Estimated Expiration: ⤷ Get Started Free
Patent: 1831447
Patent: Process for preparing substituted 5-fluoro-1H-pyrazolopyridines
Estimated Expiration: ⤷ Get Started Free
Patent: 1838993
Patent: Process for preparing substituted 5-fluoro-1H-pyrazolopyridines
Estimated Expiration: ⤷ Get Started Free
Patent: 1932465
Patent: Processes for preparing intermediates of substituted 5-fluoro-1H-pyrazolopyridines
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 14000227
Patent: METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1816203
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 6623
Patent: СПОСІБ ОДЕРЖАННЯ ЗАМІЩЕНИХ 5-ФТОР-1H-ПІРАЗОЛОПІРИДИНІВ (METHOD FOR PRODUCING SUBSTITUTED 5-FLUORO-1H-PYRAZOLOPYRIDINES)
Estimated Expiration: ⤷ Get Started Free
Patent: 9269
Patent: СПОСІБ ОДЕРЖАННЯ ПРОМІЖНИХ СПОЛУК В СПОСОБІ ОДЕРЖАННЯ ЗАМІЩЕНИХ 5-ФТОР-1Н-ПІРАЗОЛОПІРИДИНІВ
Estimated Expiration: ⤷ Get Started Free
Patent: 0278
Patent: ЗАМІЩЕНІ 5-ФТОР-1Н-ПІРАЗОЛОПІРИДИНИ В КРИСТАЛІЧНІЙ ФОРМІ
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 467
Patent: PROCEDIMIENTO PARA LA PREPARACIÓN DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Patent: 646
Patent: PROCEDIMIENTO PARA LA PREPARACIÓN DE 5-FLUORO-1H-PIRAZOLOPIRIDINAS SUSTITUIDAS
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering VERQUVO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Morocco | 34249 | ⤷ Get Started Free | |
| Spain | 2694158 | ⤷ Get Started Free | |
| Serbia | 54261 | UPOTREBA SGC STIMULATORA, SGC AKTIVATORA, POJEDINAČNO I U KOMBINACIJI SA PDE5 INHIBITORIMA ZA TRETMAN SISTEMSKE SKLEROZE (SSC) (THE USE OF SGC STIMULATORS, SGC ACTIVATORS, ALONE AND COMBINATIONS WITH PDE5 INHIBITORS FOR THE TREATMENT OF SYSTEMIC SCLEROSIS (SSC)) | ⤷ Get Started Free |
| Israel | 249669 | 5-פלואורו-h1-פיראזולופירידינים מותמרים והשימוש בהם (Substituted 5-fluoro-1h-pyrazolopyridines and use thereof) | ⤷ Get Started Free |
| Cyprus | 1116703 | ⤷ Get Started Free | |
| Ukraine | 116521 | ⤷ Get Started Free | |
| South Africa | 201208824 | THE USE OF SGC STIMULATORS,SGC ACTIVATORS,ALONE AND COMBINATIONS WITH PDE5 INHIBITORS FOR THE TREATMENT OF SYSTEMIC SCLEROSIS (SSC) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VERQUVO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2576547 | PA2021518 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: VERICIGUATAS IR JO DRUSKOS, SOLVATAI IR DRUSKU SOLVATAI ; REGISTRATION NO/DATE: EU/1/21/1561 20210716 |
| 2576547 | C02576547/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: VERICIGUAT; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 68001 22.09.2021 |
| 2576547 | 122021000048 | Germany | ⤷ Get Started Free | PRODUCT NAME: VERICIGUAT SOWIE DESSEN SALZE, SOLVATE UND SOLVATE DER SALZE; REGISTRATION NO/DATE: EU/1/21/1561 20210716 |
| 2576547 | 21C1038 | France | ⤷ Get Started Free | PRODUCT NAME: VERICIGUAT ET SES SELS, SOLVATES ET SOLVATES DES SELS; REGISTRATION NO/DATE: EU/1/21/1561 20210720 |
| 2576547 | CR 2021 00032 | Denmark | ⤷ Get Started Free | PRODUCT NAME: VERICIGUAT OG DETS SALTE, SOLVATER OG SOLVATER AF SALTENE; REG. NO/DATE: EU/1/21/1561 20210720 |
| 2576547 | C20210029 00408 | Estonia | ⤷ Get Started Free | PRODUCT NAME: VERITSIGUAAT;REG NO/DATE: EU/1/21/1561 20.07.2021 |
| 2576547 | PA2021518,C2576547 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: VERICIGUATAS IR JO DRUSKOS, SOLVATAI IR DRUSKU SOLVATAI ; REGISTRATION NO/DATE: EU/1/21/1561 20210716 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for VERQUVO (sacitoclax)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
