Acetaminophen - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for acetaminophen and what is the scope of patent protection?
Acetaminophen
is the generic ingredient in one hundred and eight branded drugs marketed by Ortho Mcneil Pharm, Rising, Aspiro, B Braun Medical Inc, Baxter Hlthcare Corp, Eugia Pharma, Fresenius Kabi Usa, Hikma, Inforlife, Mylan, Sandoz, Wockhardt Bio Ag, Zydus Pharms, Mallinckrodt Hosp, Cosette, Able, Acino Prods, Perrigo New York, Taro, Polymedica, J And J Consumer Inc, Aurobindo Pharma, Granules, Heritage Pharma, Marksans Pharma, Ohm Labs, Perrigo, Sun Pharm Inds Ltd, Aurobindo Pharma Ltd, Haleon Us Holdings, Mikart, Scherer Labs, Zevra Therap, Forest Pharms, Mallinckrodt, Dr Reddys Labs Sa, Valeant, Dunhall, Larken Labs Inc, Alvogen, Halsey, Lgm Pharma, Ne Rx Pharma, Quagen, Senores Pharms, Watson Labs, Mayrand, Shire, Aurolife Pharma Llc, Gilbert Labs, Graham Dm, Key Therap, Lannett Co Inc, Novast Labs, Nuvo Pharms Inc, Us Chem, Abhai Llc, Actavis Labs Ut Inc, Hikma Pharms, Mirror Pharms Llc, Nesher Pharms, Specgx Llc, Strides Pharma, Sun Pharm Industries, Vintage Pharms, Hikma Intl Pharms, Nostrum Labs Inc, Wraser Pharms Llc, Pharm Res Assoc, Leitner Pharms, Boca Pharma Llc, West-ward Pharm Corp, Novartis, Teva, Robins Ah, Solvay, ACI, Actavis Mid Atlantic, Akorn, Chartwell, Chartwell Molecular, Dava Pharms Inc, Genus Lifesciences, Valeant Pharms Llc, Am Therap, Amneal Pharms Ny, Duramed Pharms Barr, Elite Labs Inc, Everylife, Fosun Pharma, Kv Pharm, Lederle, Mutual Pharm, Puracap Pharm, Purepac Pharm, Rhodes Pharms, Roxane, Superpharm, Usl Pharma, Valeant Pharm Intl, Vitarine, Warner Chilcott, Watson Labs Florida, Wes Pharma Inc, Whiteworth Town Plsn, Carnrick, Glaxosmithkline, Vangard, Janssen Pharms, Schering Plough, Cent Pharms, Ivax Pharms, Genus, Mallinckrodt Inc, Pharm Assoc, Tris Pharma Inc, Cypress Pharm Inc, Ucb Inc, Ascher, Actavis Labs Fl Inc, Amneal Pharms, Apil, Ascent Pharms Inc, Barr, Caraco, Cerovene Inc, Epic Pharma Llc, Novel Labs Inc, Prinston Inc, Ranbaxy, Ranbaxy Labs Ltd, Sun Pharm Inds Inc, Upsher Smith Labs, Vintage Pharms Llc, Abana, Abbott, Abbvie, Bionpharma, Glenmark Pharms Ltd, L Perrigo Co, Aft Pharms Ltd, Actavis Elizabeth, Bristol Myers Squibb, Endo Operations, Gavis Pharms, Sanofi Aventis Us, Aaipharma Llc, Xanodyne Pharm, Cornerstone, Ivax Sub Teva Pharms, Mirror Pharms, Mylan Pharms Inc, Wockhardt Ltd, Alkem Labs Ltd, Chartwell Rx, Graviti Pharms, Macleods Pharms Ltd, Micro Labs Ltd India, and Zydus Pharms Usa Inc, and is included in five hundred and twenty-seven NDAs. There are thirty patents protecting this compound and one Paragraph IV challenge. Additional information is available in the individual branded drug profile pages.Acetaminophen has twenty-one patent family members in thirteen countries.
There are sixty-six drug master file entries for acetaminophen. Ninety-four suppliers are listed for this compound. There is one tentative approval for this compound.
Summary for acetaminophen
| International Patents: | 21 |
| US Patents: | 30 |
| Tradenames: | 108 |
| Applicants: | 159 |
| NDAs: | 527 |
| Drug Master File Entries: | 66 |
| Finished Product Suppliers / Packagers: | 94 |
| Raw Ingredient (Bulk) Api Vendors: | 178 |
| Clinical Trials: | 1,308 |
| Patent Applications: | 5,362 |
| Formulation / Manufacturing: | see details |
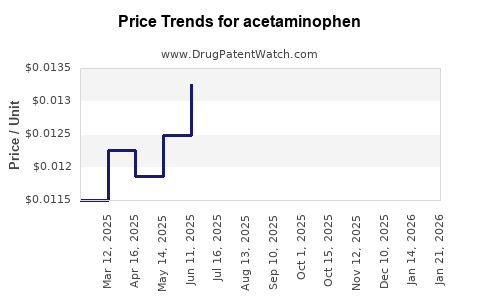
| Drug Prices: | Drug price trends for acetaminophen |
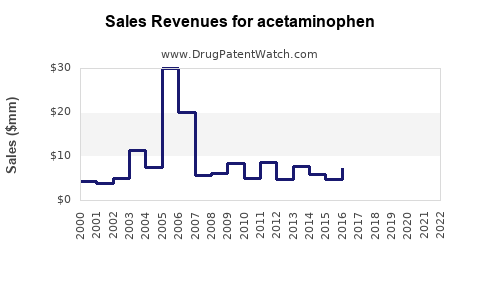
| Drug Sales Revenues: | Drug sales revenues for acetaminophen |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for acetaminophen |
| What excipients (inactive ingredients) are in acetaminophen? | acetaminophen excipients list |
| DailyMed Link: | acetaminophen at DailyMed |
Recent Clinical Trials for acetaminophen
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University Hospital "Sestre Milosrdnice" | N/A |
| Jessyka Lighthall | Phase 3 |
| Jiangsu HengRui Medicine Co., Ltd. | Phase 1 |
Generic filers with tentative approvals for ACETAMINOPHEN
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Medical Subject Heading (MeSH) Categories for acetaminophen
Paragraph IV (Patent) Challenges for ACETAMINOPHEN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| OFIRMEV | Injection | acetaminophen | 1000 mg/100 mL (10 mg/mL) | 022450 | 1 | 2011-04-07 |
US Patents and Regulatory Information for acetaminophen
Expired US Patents for acetaminophen
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| J And J Consumer Inc | TYLENOL | acetaminophen | TABLET, EXTENDED RELEASE;ORAL | 019872-001 | Jun 8, 1994 | ⤷ Sign Up | ⤷ Sign Up |
| J And J Consumer Inc | TYLENOL | acetaminophen | TABLET, EXTENDED RELEASE;ORAL | 019872-001 | Jun 8, 1994 | ⤷ Sign Up | ⤷ Sign Up |
| Mallinckrodt Hosp | OFIRMEV | acetaminophen | SOLUTION;INTRAVENOUS | 022450-001 | Nov 2, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| J And J Consumer Inc | TYLENOL | acetaminophen | TABLET, EXTENDED RELEASE;ORAL | 019872-001 | Jun 8, 1994 | ⤷ Sign Up | ⤷ Sign Up |
| Mallinckrodt Hosp | OFIRMEV | acetaminophen | SOLUTION;INTRAVENOUS | 022450-001 | Nov 2, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for acetaminophen
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2243477 | Paracétamol destiné à l'administration parentérale (Paracetamol for parenteral application) | ⤷ Sign Up |
| European Patent Office | 2421522 | PARACÉTAMOL DESTINÉ À L'ADMINISTRATION PARENTÉRALE (PARACETAMOL FOR PARENTERAL ADMINISTRATION) | ⤷ Sign Up |
| Poland | 2421522 | ⤷ Sign Up | |
| Japan | 5909750 | ⤷ Sign Up | |
| China | 102421426 | Paracetamol for parenteral administration | ⤷ Sign Up |
| Australia | 2010238854 | Paracetamol for parenteral administration | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |