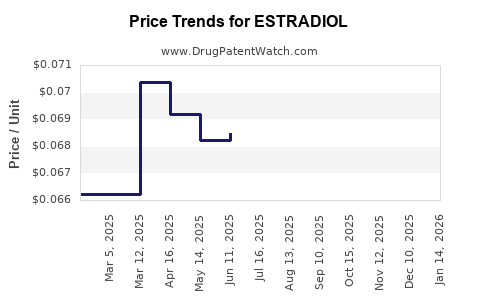
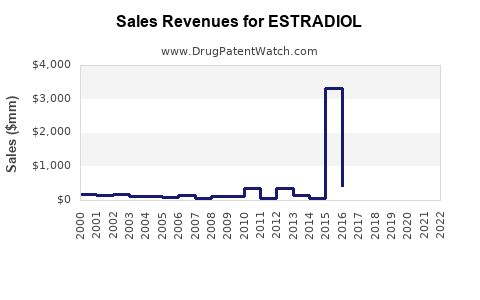
Last updated: July 27, 2025
Introduction
Estradiol, a potent estrogen hormone primarily used in hormone replacement therapy (HRT), contraception, and transgender healthcare, has long maintained a significant position within the global pharmaceutical landscape. Its market dynamics are shaped by demographic shifts, regulatory environments, technological advancements, and evolving therapeutic demands. This analysis offers a comprehensive view of recent trends, competition, regulatory factors, and forecasted financial trajectories shaping the future of estradiol-based therapeutics.
Market Overview and Key Segments
The global estradiol market comprises various formulations, including oral tablets, transdermal patches, gels, creams, vaginal rings, and injections. Each segment addresses specific clinical needs, with transdermal delivery gaining prominence due to improved bioavailability and reduced hepatic first-pass metabolism (1).
According to reports, the global estrogen therapy market, driven chiefly by estradiol formulations, was valued at approximately USD 1.7 billion in 2022 and is poised for a compound annual growth rate (CAGR) of around 4-6% over the next five years (2). The rise is notably linked to increasing aging populations, greater awareness of menopause management, and expanding indications in transgender healthcare.
Drivers of Market Growth
Demographic and Epidemiological Factors
The aging global population significantly propels estradiol demand. Women aged 50+ are more susceptible to menopause-related hormonal imbalances, requiring hormone replacement therapies. The WHO estimates a global menopausal population exceeding 1 billion by 2030, underpinning sustained market growth (3).
Furthermore, rising awareness and acceptance of gender-affirming therapies in transgender individuals bolster demand, especially for estradiol in combination with testosterone blockers (4).
Advancements in Drug Delivery and Formulation Technologies
Innovative delivery systems, such as bioidentical estradiol compounded creams and novel patches, enhance efficacy and patient compliance. The development of bioidentical hormones aligning more closely with endogenous human estrogen improves tolerability and safety profiles, spurring market expansion (5).
Regulatory Environment and Patent Expirations
Patent expirations for leading branded products, coupled with regulatory approvals for generic versions, amplify market penetration. The entry of generics typically reduces costs, enlarging access, especially in emerging markets (6).
Conversely, stringent regulatory landscapes in regions like the US (FDA) and Europe (EMA) impose rigorous safety standards, influencing formulation approvals and market readiness.
Evolving Clinical Guidelines and Safety Perceptions
Heightened awareness around hormone therapy-related risks—such as thromboembolism, breast cancer, and cardiovascular events—has led to refined prescribing guidelines. Emphasis on personalized medicine and tailored dosages aims to optimize benefits while mitigating adverse effects, influencing market dynamics (7).
Competitive Landscape
Major pharmaceutical companies such as Pfizer, Novo Nordisk, and Teva Pharmaceuticals hold prominent positions, offering branded estradiol products supported by extensive marketing and R&D capabilities. The generic market segment continues to grow, intensifying price competition and accessibility.
Additionally, niche players focus on innovative delivery platforms, including microneedle systems and bioengineered estrogens, aiming to capture unmet clinical needs. Strategic alliances, licensing agreements, and acquisitions further shape competitive dynamics.
Regulatory and Legal Factors
Regulatory agencies have increased monitoring of hormone therapies due to safety concerns. The FDA's 2017 guidance on compounded hormone therapies emphasizes ensuring quality and consistency, influencing market practices (8). Countries tightening regulatory controls may delay or restrict approval pathways, impacting revenue forecasts.
Legal disputes over off-label uses and patent rights also influence market stability. Companies investing heavily in R&D for biosimilar estradiol versions anticipate alternative pathways to extend market share amid patent cliffs.
Financial Trajectory
Revenue Forecasts and Market Growth Potential
Estrodiol sales are expected to grow at a CAGR of approximately 4-6% through 2030, driven by demographic trends and expanding clinical applications (2). The regional analysis indicates highest growth potential in Asia-Pacific, Latin America, and the Middle East, where healthcare access expands and regulatory frameworks evolve.
Impact of Generics and Biosimilars
The entrance of biosimilars and generics is projected to reduce prices by 20-40%, potentially expanding market reach but constraining profit margins for branded products (6). The transition emphasizes cost-effective manufacturing and strategic pricing.
Emerging Markets and Price Sensitivity
Emerging economies harbor substantial growth prospects due to increasing healthcare infrastructure investments and rising middle-class populations. However, price sensitivity and regulatory hurdles may temper growth rates, necessitating adaptive marketing strategies.
Investment Trends
Investments in innovative delivery systems and research into bioidentical hormones signal a shift toward value-added products with improved safety, further influencing revenue streams. Mergers and acquisitions aiming to acquire specialized formulations could alter the competitive and financial landscape significantly.
Risks and Challenges
- Safety and Regulatory Scrutiny: Heightened vigilance over hormone-related adverse effects may result in tighter controls, impacting product availability.
- Market Saturation: Mature markets in North America and Europe could face stagnation, requiring diversification into newer formulations or indications.
- Patent Challenges: Expiration of key patents could catalyze fierce price competition from generics.
- Societal and Cultural Factors: Variations in acceptance of hormone therapies globally can influence uptake rates.
Future Outlook
The estradiol market's trajectory appears resilient, buoyed by demographic shifts and expanding indications. Technological innovations, strategic market entries, and regulatory navigation will define the horizon. Companies prioritizing safety profiles and personalized treatment modalities will likely sustain competitive advantages and profitability.
Key Takeaways
- Demographic Trends: Aging populations and increasing transgender healthcare needs underpin sustained demand.
- Innovation Drive: Delivery systems enhancing compliance and safety are central to market expansion.
- Regulatory Landscape: Vigilance regarding safety profiles influences approval pathways and sales strategies.
- Price Dynamics: Generics and biosimilars are reshaping profit margins and accessibility.
- Emerging Markets: High growth potential, contingent on regulatory and economic factors.
FAQs
-
What are the primary therapeutic applications of estradiol?
Estradiol is predominantly used in hormone replacement therapy for menopausal women, in contraceptive formulations, and in transgender hormone therapies.
-
How do patent expirations influence the estradiol market?
Patent expirations open the market to generic competitors, reducing prices and increasing accessibility but pressuring branded product revenues.
-
What technological innovations are shaping estradiol delivery?
Innovations include transdermal patches, bioidentical creams, gels, vaginal rings, and microneedle patches, which improve bioavailability and patient adherence.
-
What safety concerns impact regulatory approval for estradiol therapies?
Risks such as blood clots, breast cancer, and cardiovascular events lead to stringent safety evaluations, influencing approval and prescribing practices.
-
What is the outlook for estradiol's market growth beyond 2025?
The market is expected to grow steadily at a CAGR of 4-6%, driven by demographic trends, clinical innovation, and expanding global access, with emerging markets offering significant opportunities.
References
- [1] International Journal of Women's Health, 2020. Advances in Estrogen Delivery Technologies.
- [2] Mordor Intelligence, Estrogen Therapy Market Reports, 2022.
- [3] WHO, Menopause and the Global Aging Population, 2021.
- [4] Journal of Clinical Endocrinology & Metabolism, 2021. Transgender Hormone Therapy Market Overview.
- [5]Pharmaceutical Technology, Innovations in Bioidentical Hormone Formulations, 2022.
- [6] IQVIA, Impact of Patent Expirations on Hormone Therapies, 2021.
- [7] FDA, Hormone Therapy Safety Guidelines, 2019.
- [8] FDA, Guidance on Compounded Hormone Replacement Therapies, 2017.