Introduction: The Unseen Engine of Modern Healthcare
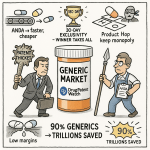
The generic drug industry operates at the center of a fundamental paradox. It is the unseen engine of modern healthcare, a force of immense societal value that ensures broad access to essential medicines. In the United States alone, generic drugs account for approximately 90% of all prescriptions filled, yet they represent a mere 13.1% of the nation’s total expenditure on prescription drugs.1 This staggering disparity translates into monumental savings: $445 billion for the U.S. healthcare system in 2023 and an estimated $3.1 trillion over the preceding decade.3 These are not just abstract figures; they represent tangible benefits for patients, employers, and taxpayers, enabling greater medication adherence, reducing the burden of chronic disease, and promoting health equity.6
However, this value creation is born from a hyper-competitive, low-margin environment where long-term survival is a constant struggle.7 The very mechanism that generates these savings—intense price competition that erodes the monopoly profits of brand-name drugs—simultaneously threatens the sustainability of the generic manufacturers themselves.9 The business model is predicated on an inherently unstable foundation; it thrives by systematically dismantling the very market exclusivity that funds the next wave of innovator drugs it will eventually need to copy. This perpetual cycle of creative destruction is both the industry’s greatest public health contribution and its most profound strategic vulnerability. The tension between rewarding innovation and ensuring affordable access defines the entire pharmaceutical landscape, creating a fragile ecosystem where policies that strengthen one side invariably weaken the other.
The common perception of generic drugs as simple “copies” of their brand-name counterparts belies the sophisticated scientific and strategic reality of their development.10 Modern generic development, particularly with the rise of more complex products, is a gauntlet of scientific reverse-engineering, rigorous regulatory scrutiny, and high-stakes legal battles.11 The journey from identifying a brand-name drug’s expiring patent to placing a bioequivalent alternative on the pharmacy shelf is a complex, multi-year endeavor demanding expertise across chemistry, manufacturing, regulatory affairs, and intellectual property law.
This report deconstructs the entire generic drug lifecycle, from the foundational legal frameworks to the commercial realities of the market. Its central thesis is that in the contemporary generic drug industry, market leadership and long-term viability are achieved not through manufacturing prowess alone, but through the sophisticated mastery of turning information into a decisive competitive advantage.11 Patent databases, regulatory filings, and market data are no longer just sources of information; they are strategic assets. Platforms like
DrugPatentWatch have become indispensable tools in this process, enabling companies to transform raw data into actionable intelligence. By dissecting each stage of the development process, this report will illuminate the strategies, challenges, and future trends that define the unseen engine of modern medicine.
Section 1: The Blueprint for Competition – Deconstructing the Hatch-Waxman Act
The modern generic drug industry in the United States was born from a landmark piece of legislation: the Drug Price Competition and Patent Term Restoration Act of 1984, known colloquially as the Hatch-Waxman Act.13 Prior to its passage, the generic market was nascent and inefficient, with generic products accounting for a mere 19% of prescriptions.11 The Act was not simply a pro-generic law; it was a carefully calibrated “compromise” designed to strike a delicate “balance between two potentially competing policy interests—inducing pioneering development of pharmaceutical formulations and methods and facilitating efficient transition to a market with low-cost, generic copies”.15 Understanding this foundational balance is critical to comprehending the strategic dynamics of the entire industry. The Act rests on four interconnected pillars that collectively created the framework for today’s competitive landscape.
Pillar 1: The Abbreviated New Drug Application (ANDA) Pathway
The central mechanism for generic drug approval established by Hatch-Waxman is the Abbreviated New Drug Application (ANDA) pathway, codified under section 505(j) of the Federal Food, Drug, and Cosmetic Act.12 The application is “abbreviated” because it liberates generic manufacturers from the need to conduct their own expensive and time-consuming preclinical and clinical trials to prove safety and effectiveness.10 Instead, the ANDA pathway allows a generic applicant to rely on the FDA’s previous finding that the innovator’s product—the Reference Listed Drug (RLD)—is safe and effective.18 The generic applicant’s primary scientific burden is to demonstrate that its product is “bioequivalent” to the RLD, meaning it works in the same way and provides the same clinical benefit.11 This provision dramatically lowered the barriers to entry, reducing development costs and shortening approval timelines from the 9-15 years typical for a new drug to a more manageable 18-36 months.15
Pillar 2: Incentives for Innovators – Patent Term Restoration & Exclusivities
To balance the new competitive threat posed by the ANDA pathway, the Act provided two crucial protections for innovator companies to ensure that the financial incentives for R&D remained intact.
First, the Act created a provision for Patent Term Extension, allowing brand-name companies to restore a portion of the patent life that was consumed during the lengthy FDA regulatory review process.14 This ensures that the effective period of market exclusivity is not unduly eroded by administrative delays.
Second, and perhaps more importantly, the Act codified several forms of Regulatory Exclusivity. These are periods of market protection, granted by the FDA, that are entirely independent of a drug’s patent status. They can run concurrently with patents or provide protection even after patents have expired. Key exclusivities include:
- Five-Year New Chemical Entity (NCE) Exclusivity: This is a cornerstone of innovator protection. For a drug containing an active ingredient never before approved by the FDA, the agency is prohibited from accepting an ANDA submission for five years from the date of the innovator’s approval. If the generic applicant plans to challenge a patent, this period is shortened to four years.12
- Three-Year New Clinical Investigation Exclusivity: This exclusivity is granted for applications or supplements that contain reports of new clinical investigations (other than bioavailability studies) that were essential to the approval. This often applies to new formulations, new dosage strengths, or new indications for an already-approved drug. It prevents the FDA from approving an ANDA for that specific change for three years.12
- Pediatric and Orphan Drug Exclusivities: To encourage development for underserved populations, the Act provides additional incentives. Pediatric exclusivity adds six months of protection to all existing patents and exclusivities for a drug if the sponsor conducts requested studies in children.15 Orphan drug exclusivity provides seven years of market protection for drugs developed to treat rare diseases (affecting fewer than 200,000 people in the U.S.), during which the FDA cannot approve another application for the same drug for the same indication unless the new product is clinically superior.15
Pillar 3: The “Safe Harbor” Provision (21 U.S.C. § 271(e)(1))
A crucial, though less publicly known, provision of Hatch-Waxman is the “safe harbor”.15 Before the Act, any use of a patented drug for development purposes, including the clinical studies needed to prepare a generic application, constituted patent infringement. This created a de facto period of market exclusivity for brand-name drugs
after their patents expired, as generic companies could only begin their development work upon patent expiry.15 The safe harbor provision eliminated this barrier by shielding generic companies from patent infringement lawsuits for activities that are “solely for uses reasonably related to the development and submission of information under a Federal law which regulates the manufacture, use or sale of drugs”.15 The Supreme Court has interpreted this clause broadly, allowing generic firms to conduct all necessary development and testing work before the innovator’s patents expire, ensuring they can be ready to launch their products on or shortly after the day of patent loss.15
Pillar 4: The Orange Book and Patent Certifications
To manage the complex interplay of patents and generic applications, the Hatch-Waxman Act formalized the FDA’s publication of “Approved Drug Products with Therapeutic Equivalence Evaluations,” now universally known as the Orange Book.11 This publication serves as the definitive public record of approved drugs, their therapeutic equivalence ratings, and, critically, the patents that brand-name companies assert cover their products.22
When a generic company files an ANDA, it must review the Orange Book and make a patent certification for each patent listed for the RLD. This certification is a legal declaration that sets the stage for potential litigation. There are four types of certifications, but the most strategically important is the Paragraph IV certification, in which the generic applicant declares that, in its opinion, the brand’s patent is invalid, unenforceable, or will not be infringed by the proposed generic product.22 As will be explored in detail later, this certification is the trigger for the high-stakes legal battles that define the industry.
The intricate design of the Hatch-Waxman Act, with its interlocking provisions, created a dynamic and often contentious system. The following table illustrates the fundamental balance of power the Act established.
| Provisions Favoring Generic Competition | Provisions Favoring Innovator Protection |
| Abbreviated New Drug Application (ANDA) Pathway | Patent Term Restoration |
| Bioequivalence as the Standard for Approval | Five-Year New Chemical Entity (NCE) Exclusivity |
| “Safe Harbor” Provision for Pre-Expiration Development | Three-Year New Clinical Investigation Exclusivity |
| 180-Day Exclusivity Incentive for Patent Challengers | Pediatric and Orphan Drug Exclusivities |
| Paragraph IV Patent Challenge Mechanism | 30-Month Stay of ANDA Approval During Litigation |
Section 2: The Pre-Market Gauntlet – Strategic Product Selection and Competitive Intelligence
The single most critical decision a generic drug company makes is not in the laboratory or on the manufacturing floor, but in the boardroom during the strategic selection of its next product target.11 This decision dictates the entire subsequent pathway, influencing research and development costs, regulatory complexity, litigation risk, and ultimate commercial viability. The modern paradigm for product selection has evolved far beyond simply identifying a drug’s patent expiration date. It is now a holistic, integrated risk assessment that balances intellectual property intelligence with deep technical and commercial analysis.
Decoding the Patent Landscape
The process invariably begins with the “patent cliff”—the point at which a blockbuster brand-name drug loses its primary patent protection, opening the door to generic competition.5 However, this is rarely a single, clean date. Innovator companies strategically build a “patent thicket” around their most valuable products, a layered defense of multiple patent types designed to frustrate and delay generic entry.12 A thorough analysis must dissect this thicket, which typically includes:
- Composition of Matter Patents: The core patent protecting the active pharmaceutical ingredient (API) itself. This is the strongest and most fundamental form of protection.11
- Formulation Patents: Covering the specific combination of active and inactive ingredients or the drug’s delivery system (e.g., an extended-release tablet).11
- Method of Use Patents: Protecting a specific, approved indication for the drug.24
- Process Patents: Covering the specific method of manufacturing the drug.24
Navigating this complexity requires moving beyond the surface-level data in the FDA’s Orange Book to a more dynamic and integrated view of the intellectual property landscape.
Leveraging Competitive Intelligence Platforms: The Role of DrugPatentWatch
This is where sophisticated competitive intelligence platforms like DrugPatentWatch become indispensable strategic tools.27 These platforms aggregate and integrate data from the FDA, the U.S. Patent and Trademark Office (USPTO), international patent offices, and court records, transforming disparate data points into a cohesive intelligence picture that informs critical business decisions.27
Strategic use cases for such platforms are manifold:
- Comprehensive Portfolio Management: By providing a holistic view of patent expirations, regulatory exclusivities, and potential market entry dates, these tools allow portfolio managers to anticipate future opportunities, forecast revenue streams, and proactively identify potential generic targets.27
- In-depth Competitor Analysis: A key function is tracking litigation. Platforms like DrugPatentWatch allow a company to see which competitors have already filed Paragraph IV challenges against a target drug, assess the historical success rates of those challengers, and monitor the progress of ongoing litigation.24 This intelligence is vital for determining if the lucrative 180-day first-to-file exclusivity is still available or if the market will be crowded upon entry.
- Formulation and Sourcing Intelligence: By delving into the details of patents and Drug Master Files (DMFs), companies can gain crucial insights into an innovator’s formulation strategies, identifying the specific excipients and manufacturing processes used.27 This can significantly accelerate the reverse-engineering process. The platform can also help identify potential suppliers of the raw API, a critical step in securing the supply chain.27
Beyond Patents: A Multi-Factor Commercial Assessment
A robust intellectual property analysis is necessary but not sufficient. The most successful generic companies layer a rigorous commercial and technical assessment on top of the patent intelligence to build a complete business case.11 This multi-factor analysis includes:
- Market Size and Erosion Modeling: The first question is always about the size of the prize. What are the annual sales of the brand-name drug? This figure must then be subjected to a realistic price erosion model. The number of expected competitors is the key variable; a market with only one or two generics will see modest price declines, while a market with six or more will see prices plummet by over 95%, rapidly commoditizing the product.11
- Manufacturing and Regulatory Complexity: Not all generics are created equal. Is the target a simple, immediate-release oral solid tablet, or is it a “complex generic”? Complex products—such as long-acting injectables, metered-dose inhalers, transdermal patches, or drugs with complex APIs—are significantly more difficult and expensive to develop.31 They may require more extensive and uncertain bioequivalence studies, and the FDA may not have issued a clear Product-Specific Guidance (PSG) for development, increasing regulatory risk.8
- First-to-File (FTF) Potential: The analysis of litigation and patent strength feeds directly into this crucial commercial question. Is there a viable opportunity to be the first applicant to challenge a weak patent? Securing the 180-day exclusivity period can be the difference between a highly profitable product and a low-margin commodity, and is often the primary driver for pursuing a high-risk patent challenge.24
The interplay of these factors reveals that the most attractive target is not always the one with the largest market size or the soonest patent expiry. A blockbuster drug facing a “patent cliff” might be a value trap if it is technically simple to replicate, guaranteeing a flood of competitors and rapid price erosion. Conversely, a product with a dense patent thicket and moderate sales might represent a superior opportunity if the patents are vulnerable to challenge, the product is technically complex (creating high barriers to entry), and the 180-day exclusivity prize is attainable. This shift from a singular focus on IP expiration to an integrated risk assessment is the hallmark of a mature and sophisticated generic strategy. The following framework synthesizes this complex decision-making process into an actionable tool.
| Analysis Domain | Critical Questions | Key Tools & Data Sources |
| Intellectual Property Landscape | What is the last-to-expire patent? Is there a “patent thicket”? How strong are the patents (composition of matter vs. method of use)? Who are the patent holders? | FDA Orange Book, USPTO Database, DrugPatentWatch |
| Litigation & Exclusivity | Are there existing Paragraph IV challenges? Who are the filers? What is their historical success rate? Is the 180-day exclusivity available? | DrugPatentWatch, U.S. Court Dockets (PACER), Legal Databases |
| Regulatory & Technical Complexity | Is this a complex generic? Does an FDA Product-Specific Guidance (PSG) exist? What are the likely bioequivalence study requirements? How complex is the formulation to reverse-engineer? | FDA Website (PSGs), Scientific Literature, Internal R&D Expertise |
| Commercial Viability | What is the brand’s market size? What is the projected price erosion curve based on likely competition? What is the expected number of competitors? Are there reimbursement challenges? | IQVIA/IMS Health Market Data, Payer Formularies, Financial Analyst Reports |
Section 3: The Science of Replication – From Reverse Engineering to a Validated Process
Once a target drug has been strategically selected, the focus shifts from the boardroom to the laboratory. The core scientific mandate of generic drug development is replication under the principle of “sameness”.35 A generic product must be a “pharmaceutical equivalent” to the innovator’s Reference Listed Drug (RLD), containing the identical active ingredient in the same dosage form and strength, and administered by the same route.20 Crucially, it must also be “bioequivalent,” demonstrating that it performs identically in the human body.37 This process is a multi-stage scientific endeavor, moving from meticulous deconstruction to robust, large-scale production.
Step 1: De-formulation – The Art of Reverse Engineering
The development journey begins with a process of methodical deconstruction known as de-formulation, or reverse engineering.11 The objective is to create a formulation that is qualitatively (Q1) and quantitatively (Q2) the same as the RLD, meaning it contains the same ingredients in the same amounts.36
- Characterizing the RLD: The first step is to obtain and meticulously analyze samples of the RLD. This involves more than just confirming the identity of the Active Pharmaceutical Ingredient (API); it requires a deep characterization of its physical and chemical properties, such as particle size, polymorphism, and purity profile. The impurity profile of the RLD can provide valuable clues about the chemistry, manufacturing, and controls (CMC) used in its development.36
- Decoding the Recipe: The true scientific challenge lies in identifying and quantifying the various inactive ingredients, or excipients, that make up the drug product.41 Excipients are not merely fillers; they can play critical functional roles in controlling drug release, ensuring stability, or aiding in the manufacturing process.41 Painstakingly identifying this complete formulation “recipe” is the essence of de-formulation.42
- The Analytical Toolbox: To achieve the necessary precision and reproducibility, scientists employ a battery of sophisticated analytical techniques. High-Performance Liquid Chromatography (HPLC) is used to separate, identify, and quantify components. Mass Spectrometry (MS) provides highly accurate molecular weight information to confirm identities. Fourier-Transform Infrared (FTIR) Spectroscopy helps identify functional groups and characterize the solid-state properties of the drug and excipients.42
Step 2: Formulation Development and Quality by Design (QbD)
With a complete understanding of the RLD’s composition, the generic formulator begins the work of creating a bioequivalent product. The modern approach to this task has moved beyond empirical trial-and-error to a more systematic, science- and risk-based framework known as Quality by Design (QbD).36
- Building Quality In: The philosophy of QbD is to build quality into the product and process from the outset, rather than testing for it at the end. This proactive approach leads to a more robust and consistent manufacturing process.40
- QbD in Practice: The process begins with defining a Quality Target Product Profile (QTPP), which outlines the desired characteristics of the final drug product (e.g., identity, assay, dissolution profile). From the QTPP, scientists identify the Critical Quality Attributes (CQAs)—physical, chemical, or biological attributes that must be controlled to ensure the desired product quality. Through risk assessment, they then identify the Critical Process Parameters (CPPs)—manufacturing variables that can affect the CQAs. Finally, they establish a Design Space, which is the multidimensional combination of input variables (e.g., material attributes) and process parameters that has been demonstrated to provide assurance of quality.40
- Innovations in Formulation: While the primary goal is replication, the generic industry is increasingly investing in formulation technologies to create “value-added” or “super generics”.35 These products go beyond simple bioequivalence to offer tangible improvements, such as enhanced bioavailability through nanotechnology or more convenient administration via complex delivery systems.41
Step 3: Analytical Method Development and Validation
Running parallel to formulation development is the critical task of creating and validating the analytical methods that will be used to test the drug product throughout its lifecycle.44 These methods are essential for ensuring the identity, strength, quality, purity, and potency (ISQPP) of every batch produced.45
The development and validation of these methods are governed by rigorous international standards, primarily the guidelines from the International Council for Harmonisation (ICH). The recently updated ICH Q2(R2) guideline on Validation of Analytical Procedures and the new Q14 guideline on Analytical Procedure Development emphasize a lifecycle approach.46 This represents a shift from a prescriptive, checklist-based validation to a more flexible, science- and risk-based framework. Regulators like the FDA now expect companies to demonstrate a deep understanding of their analytical methods, including the sources of variability and the robustness of the procedure when faced with minor changes in parameters.46
Step 4: Manufacturing Scale-Up and Good Manufacturing Practices (GMP)
The final stage of technical development is to translate the successfully developed lab-scale formulation into a robust, consistent, and compliant commercial-scale manufacturing process.44 This process of scale-up and technology transfer is fraught with challenges and must be managed through a formal process validation framework, which typically involves three stages: Process Design, Process Qualification, and Continued Process Verification.11
All pharmaceutical manufacturing, whether for brand or generic products, must adhere to a stringent set of quality standards known as Good Manufacturing Practices (GMP), or cGMP for “current” Good Manufacturing Practices.48 These are the minimum standards that a manufacturer must meet to ensure that their medicines are of consistent high quality, are appropriate for their intended use, and meet all requirements of the marketing authorization.48 Regulatory bodies like the FDA in the U.S. and the EMA in Europe, along with the World Health Organization (WHO), coordinate and enforce these standards through regular facility inspections.48 Maintaining compliance with these global standards is a significant operational and financial undertaking; for example, the Indian pharmaceutical industry, a major global supplier, spends nearly $1 billion annually just to maintain its US FDA-approved facilities.7
The traditional view of Chemistry, Manufacturing, and Controls (CMC) as a purely technical, regulatory-driven cost center is becoming obsolete. In today’s market, CMC has been weaponized into a powerful strategic tool. The hyper-commoditization of simple oral solids, which has driven margins to “wafer-thin” levels, is forcing a strategic migration toward more complex products.5 Success in these higher-value segments—such as complex generics and biosimilars—is defined almost entirely by CMC expertise. The ability to reverse-engineer a complex formulation, master a difficult-to-control manufacturing process, or develop a novel drug delivery system creates formidable barriers to entry that price alone cannot overcome.32 Furthermore, recent industry-wide quality crises, such as the issue of nitrosamine impurities, have demonstrated that companies with superior analytical and process control capabilities can navigate these challenges and maintain market supply while less capable competitors are forced to conduct costly recalls or withdraw from the market entirely.8 In this context, investing in world-class CMC capabilities is no longer just about ensuring quality; it is about building a defensible competitive moat, enabling a company to tackle products others cannot, justify higher prices for value-added generics, and transform a traditional cost center into a driver of profit and long-term strategy.
Section 4: The Gateway to Market – Navigating Global Regulatory Pathways
Securing regulatory approval is the final and most crucial gate a generic drug must pass before it can reach patients. The global regulatory landscape, however, is not a single, harmonized system but a complex maze of divergent pathways, timelines, and scientific requirements.8 A generic company with global ambitions must master the intricacies of each major market, as a failure to align development with specific regional requirements can result in duplicated efforts, costly delays, and staggered launches that undermine the economics of a global program.8 The two most significant regulatory systems are those of the United States and the European Union.
The U.S. Pathway: The Abbreviated New Drug Application (ANDA) Process
In the United States, the exclusive pathway for generic drug approval is the Abbreviated New Drug Application (ANDA), submitted to the FDA’s Center for Drug Evaluation and Research (CDER).17 The entire process is now managed through electronic submissions in the Electronic Common Technical Document (eCTD) format.51 The ANDA lifecycle proceeds through several distinct phases:
- Pre-ANDA Phase: Prudent applicants often engage with the FDA before submission through pre-ANDA meetings or controlled correspondence to clarify scientific and regulatory requirements, especially for complex products.31
- Submission and Filing Review: Upon receipt, the FDA conducts an initial administrative and completeness review to determine if the ANDA is acceptable for filing.52
- Substantive Review: Once filed, the application undergoes a comprehensive scientific review by multidisciplinary teams within the FDA, assessing all aspects of the submission, including Chemistry, Manufacturing, and Controls (CMC), labeling, and bioequivalence.18 During this phase, the FDA may issue Information Requests (IRs) for clarification or a Discipline Review Letter (DRL) detailing deficiencies in a specific area.52
- FDA Action: Following the review, the FDA will take one of several actions. If the ANDA has deficiencies, the agency will issue a Complete Response Letter (CRL), outlining all issues that must be resolved before approval can be granted.18 If the ANDA meets all scientific and regulatory requirements, the FDA will issue either a Final Approval (AP) letter, allowing the product to be marketed, or a Tentative Approval (TA) letter if the ANDA is approvable but blocked from marketing by unexpired patents or exclusivities.52 The entire process, governed by goals set under the Generic Drug User Fee Amendments (GDUFA), typically takes around 30 months, although this can vary significantly.16
The Scientific Cornerstone: Proving Bioequivalence (BE)
The heart of every ANDA is the demonstration of bioequivalence.52 The FD&C Act defines a generic as bioequivalent if “the rate and extent of absorption of the drug do not show a significant difference from the rate and extent of absorption of the listed drug”.52 This ensures the generic product delivers the same amount of API to the site of action over the same time course, guaranteeing therapeutic equivalence.17 The method for demonstrating BE depends heavily on the nature of the drug product.
- Standard Pharmacokinetic (PK) Studies: For most systemically absorbed drugs (e.g., oral tablets), BE is demonstrated through a randomized, crossover clinical study in a small number of healthy volunteers (typically 24-36).12 Subjects receive both the generic (test) and the RLD (reference) product, with a washout period in between. Blood samples are taken at various time points to measure the drug’s concentration, and two key PK parameters are calculated:
Cmax (the maximum concentration achieved) and AUC (the area under the concentration-time curve, representing total drug exposure).11 For approval, the 90% confidence interval of the geometric mean ratio (Test/Reference) for both
Cmax and AUC must fall entirely within the acceptance range of 80.00% to 125.00%.54 - Approaches for Complex Generics: Demonstrating BE for complex products is far more challenging because systemic blood levels may not be relevant to the drug’s local site of action (e.g., an eye drop or asthma inhaler).33 To guide development, the FDA issues
Product-Specific Guidances (PSGs) that recommend the most appropriate methodologies.31 These can require a “weight-of-evidence” approach that may include a combination of:
- In vitro studies (e.g., dissolution testing, particle size analysis).
- In vivo pharmacodynamic (PD) studies that measure a drug’s effect on the body.
- Comparative clinical endpoint BE studies, which are essentially small-scale efficacy trials and are significantly more expensive, time-consuming, and risky for generic firms.40
- For highly variable drugs, where meeting the standard 80-125% window is difficult due to inherent biological noise, the FDA may allow for alternative statistical approaches like Reference-Scaled Average Bioequivalence (RSABE), which widens the acceptance criteria based on the measured variability of the RLD itself.58
- Biowaivers: In certain circumstances, the FDA may waive the requirement for an in vivo BE study altogether. This “biowaiver” can be granted for certain dosage forms like parenteral or oral solutions where absorption is not a factor.59 It can also be granted for some immediate-release solid oral dosage forms based on the
Biopharmaceutics Classification System (BCS), which categorizes drugs based on their solubility and permeability characteristics.57
The European Union Pathways: A Multi-Track System
In contrast to the single-track U.S. system, the European Union offers several regulatory pathways for generic drug approval, managed through the European Medicines Agency (EMA) and national competent authorities. The choice of pathway is a key strategic decision based on the company’s marketing goals and the regulatory history of the reference product.60
- Centralized Procedure (CP): This is the pan-European route. A single application is submitted to the EMA, and if successful, results in a single marketing authorization that is valid in all 27 EU member states, as well as Iceland, Liechtenstein, and Norway.60 The CP is mandatory for certain innovative medicines (e.g., biotechnology products, cancer treatments) and for generics of products that were originally approved via the CP.62 It is an optional route for other generics, provided the applicant can demonstrate that the product constitutes a “significant therapeutic, scientific or technical innovation” or that its authorization is “in the interest of patients at Union level”.62 The assessment timeline is approximately 210 days.29
- Decentralized Procedure (DCP): This is the most common route for generics that have not yet been authorized in any EU country and are intended for marketing in more than one member state.63 The applicant submits an identical application dossier simultaneously to all chosen countries. One country is selected to act as the
Reference Member State (RMS), which takes the lead on the scientific assessment and prepares an assessment report. The other chosen countries are known as Concerned Member States (CMS).60 If all states agree, national authorizations are granted. This procedure has historically faced challenges with slow feedback from some member states and a lack of complete harmonization in the review process.67 - Mutual Recognition Procedure (MRP): This procedure is used when a generic drug already holds a national marketing authorization in one EU member state.63 That country acts as the RMS, and the company can then apply for this authorization to be “mutually recognized” by other CMSs, allowing for a phased, sequential rollout across Europe.60
The existence of these multiple, distinct pathways underscores the fragmented nature of the global market. A company’s regulatory strategy must be as sophisticated as its scientific development, tailored to the unique requirements and strategic opportunities presented by each major jurisdiction.
| Pathway | Scope of Approval | Key Requirement | Typical Timeline | Strategic Consideration |
| U.S. FDA (ANDA) | United States Market Only | Bioequivalence to U.S. RLD | ~30 months (GDUFA goals) | Single large market; unique Paragraph IV litigation and 180-day exclusivity dynamics. |
| EU EMA (Centralized) | All EU Member States | Bioequivalence to EU Reference Product | ~210 days (EMA opinion) | Broad, simultaneous market access; best for generics of centrally-approved drugs or major innovations. |
| EU National (Decentralized) | Selected EU Member States | Bioequivalence; assessment led by RMS | Varies, can be lengthy | Phased or targeted multi-country launch for generics new to the EU market. |
| EU National (Mutual Recognition) | Expansion from one EU state to others | Recognition of existing national approval | Varies | Sequential, lower-risk European expansion after securing an initial national approval. |
Section 5: The Legal Battlefield – Patent Challenges and the Race for 180-Day Exclusivity
The scientific and regulatory hurdles of generic development are paralleled by a high-stakes legal battleground defined by the Hatch-Waxman Act. This legal framework does not just permit patent challenges; it actively incentivizes them, creating a system where litigation is not an exception but a core component of business strategy for both generic and brand-name companies.
The Paragraph IV Certification: A Strategic Gambit
The catalyst for this legal warfare is the Paragraph IV certification. When filing an ANDA, a generic company must certify that, in its opinion, the patents listed in the Orange Book for the reference drug are invalid, unenforceable, or will not be infringed by its proposed product.23 This is more than a simple declaration; under U.S. law, it is considered an “artificial act of infringement”.15 This unique legal construct allows patent disputes to be litigated and resolved
before the generic product actually enters the market, providing a degree of certainty for all parties.
The process is highly formalized:
- The Notice Letter: Within 20 days of the FDA accepting its ANDA for review, the generic applicant must send a detailed notice letter to the brand manufacturer and any patent holders.22 This letter must lay out the comprehensive factual and legal basis for its Paragraph IV certification, effectively serving as the opening salvo in the potential legal conflict.24
- The 45-Day Window and 30-Month Stay: Upon receiving the notice letter, the brand company has a 45-day window to file a patent infringement lawsuit against the generic applicant.24 If a suit is filed within this period, it automatically triggers a
30-month stay of FDA approval for the ANDA.13 This stay provides the brand company with a significant period of continued market exclusivity—up to two and a half years—while the patent dispute is adjudicated in court.24
The Golden Ticket: The 180-Day Exclusivity
To encourage generics to undertake the significant risk and expense of patent litigation, Hatch-Waxman created a powerful incentive: 180 days of marketing exclusivity.23 This “golden ticket” is awarded to the “first-to-file” (FTF) ANDA applicant that contains a Paragraph IV certification.14
The financial value of this exclusivity is immense. During this 180-day period, the FDA cannot approve any subsequent ANDAs for the same drug that also contain a Paragraph IV certification.23 This effectively creates a duopoly between the brand-name drug and the first generic entrant. Freed from the pressure of mass generic competition, the FTF generic can price its product at a modest discount to the brand and capture substantial market share and revenue.24 For many of the most widely used medicines, such as atorvastatin (Lipitor) and omeprazole (Prilosec), the 180-day exclusivity has been the primary driver of patent challenges and has been directly responsible for accelerating patient access to more affordable alternatives.34
The Brand’s Counter-Offensive
Brand-name manufacturers, facing the catastrophic revenue loss of the “patent cliff,” have developed a sophisticated arsenal of legal and commercial strategies to defend their monopolies and dilute the value of generic entry.
- Authorized Generics (AGs): Perhaps the most potent counter-tactic is the launch of an authorized generic. An AG is the exact same drug product as the brand—same active and inactive ingredients, same formulation—but marketed without the brand name on the label.19 It is marketed under the brand’s original New Drug Application (NDA), often by a subsidiary of the brand company or a licensed partner.69 Crucially, because an AG is not an ANDA product, its launch is not blocked by the 180-day exclusivity. A brand company can launch an AG at any time, including on the very first day of the FTF generic’s 180-day period.26 This move immediately introduces a third competitor into the market, shattering the FTF’s duopoly and dramatically reducing its potential revenue.
- “Pay-for-Delay” Settlements: Faced with the high cost and uncertainty of litigation, many patent disputes end in a settlement. A highly controversial form of settlement is the “pay-for-delay” or “reverse payment” agreement, where the brand-name manufacturer provides a payment or other form of compensation to the generic challenger in exchange for the generic agreeing to delay its market entry until a later date.70 These agreements have been the subject of intense antitrust scrutiny from the Federal Trade Commission (FTC) and the courts. In the landmark case
FTC v. Actavis (2013), the Supreme Court ruled that such payments could violate antitrust laws.24 The FTC has estimated that these deals cost consumers and taxpayers $3.5 billion annually by keeping lower-cost alternatives off the market 72, with the cost to the federal government alone potentially reaching billions of dollars over a decade.73 - “Product Hopping” and “Patent Thickets”: Other defensive strategies include “product hopping,” where a brand company makes a minor modification to its product (e.g., changing from a tablet to a capsule) and works to switch the market to this new, separately patented version just before the original patent expires.12 The creation of dense “patent thickets” through the filing of numerous, often overlapping, secondary patents on formulations and methods of use also serves to create a legal minefield that is costly and time-consuming for generic challengers to navigate.26
The 180-day exclusivity, once viewed as the unambiguous grand prize of generic development, has thus evolved into a far more complex and risk-laden asset. Its value is no longer guaranteed. The high probability of a brand launching an authorized generic to dilute the exclusivity period means that the financial upside of winning a patent challenge is systematically diminished. Simultaneously, the legal and reputational risks associated with the settlement agreements often used to secure a market entry date have increased dramatically due to heightened antitrust enforcement. This creates a difficult strategic calculation for a potential first-filer. Litigating a patent challenge to the very end is expensive and uncertain. Settling the case may provide more certainty but carries the risk of a government investigation and further litigation. And even a decisive victory in court may lead to a prize that is immediately devalued by an AG competitor. The 180-day exclusivity is still a powerful incentive, but it is no longer a simple lottery ticket; it is a high-stakes poker hand that must be played with exceptional strategic and legal care.
Section 6: The Commercial Warzone – Pricing, Reimbursement, and Market Maturation
Upon receiving final FDA approval and navigating the legal minefield of patent litigation, a generic drug enters the commercial market—a fiercely competitive warzone governed by rapid price erosion and powerful market gatekeepers. The societal benefit of affordable medicine is forged in this environment, but it is also where the economic viability of generic manufacturing is most severely tested.
The Launch and the Price Cascade
The economic impact of generic entry is both immediate and dramatic. The relationship between the number of competitors and the price of a drug is one of the most consistent and well-documented phenomena in the pharmaceutical industry.
- Price Erosion Dynamics: The entry of the first generic competitor typically leads to a substantial price reduction, often in the range of 30% to 39% compared to the brand-name price.8 However, the true price collapse—often referred to as the “price cascade” or “price erosion”—occurs as more competitors enter the market. With just two generic competitors, the average price can fall by 54%.29 As the number of suppliers increases to six or more, the price plummets, often falling by more than 95% relative to the pre-generic entry price.29 This intense price competition is the central economic force that drives the market toward commoditization.5 In response, brand-name companies may lower their own prices to retain some market share, but they rarely match the deep discounts offered by a multi-player generic market.74
| Number of Generic Competitors | Average Price Reduction vs. Brand Price | |
| 1 | ~39% | |
| 2 | ~54% | |
| 3-5 | 70-80% | |
| 6+ | >95% | |
| Data synthesized from 8 |
The Gatekeepers: PBMs and Payers
In the U.S., market access is not simply a matter of having an approved product. It is controlled by powerful intermediaries, primarily Pharmacy Benefit Managers (PBMs) and insurance companies.76 PBMs manage prescription drug benefits on behalf of health plans, employers, and government programs. They wield immense negotiating power, with the three largest PBMs—CVS Caremark, Express Scripts, and OptumRx—controlling approximately 79% of the market.76 PBMs determine which drugs are included on a health plan’s
formulary (its list of covered drugs) and at what tier, which dictates the patient’s out-of-pocket cost or co-payment. By placing generics on lower, more favorable tiers with minimal co-pays (the average generic co-pay is around $6.16, versus $56.12 for brands), PBMs and payers create powerful financial incentives that drive patients and pharmacies to substitute generics for more expensive brand-name drugs.2
A Fragmented Global Landscape
Outside the U.S., pricing and reimbursement systems are highly fragmented, requiring generic companies to develop tailored market access strategies for each country.5
- United States: The system is largely market-based but heavily influenced by the negotiating power of PBMs. A significant recent development is the Inflation Reduction Act (IRA), which for the first time empowers the federal government (through Medicare) to directly negotiate the prices of certain high-cost drugs that lack generic or biosimilar competition. This has the potential to fundamentally alter market dynamics by lowering the brand price anchor from which generics typically discount.5
- Europe: The continent is a patchwork of national systems.
- Germany uses a reference pricing system, where the government sets a maximum reimbursement amount for a group of therapeutically similar drugs. This, combined with a tendering system for many products, creates intense and continuous downward price pressure.5
- The United Kingdom has a more market-driven approach, but high levels of generic prescribing and competition result in some of the lowest generic prices in Europe.5
- Spain employs a reference pricing system that mandates the first generic must be priced at least 40% below the originator’s price at the time of launch.61
- Asia-Pacific:
- India, known as the “pharmacy of the world,” has a market characterized by a massive volume of low-cost production and fierce domestic competition among hundreds of manufacturers.5
- China’s market has been radically reshaped by its Volume-Based Procurement (VBP) policy. Under VBP, the government organizes a national tender, guaranteeing the winning bidder(s) a massive volume of sales in the public hospital system in exchange for drastic price cuts, which have often exceeded 90%. This “winner-take-all” or “winner-take-most” system has made traditional sales and marketing efforts obsolete and has made aggressive pricing and manufacturing efficiency the sole determinants of success.5
The Sustainability Crisis: When Prices Fall Too Low
While the relentless downward pressure on prices is a victory for healthcare affordability, it has created a looming sustainability crisis for the generic industry. When prices fall below the cost of production and regulatory compliance, the business case for manufacturing a drug evaporates. This can lead to market consolidation, as smaller players are forced out, and ultimately to manufacturers discontinuing product lines altogether.7 These market withdrawals are a primary cause of
drug shortages, which disrupt patient care and impose significant costs on the healthcare system.8 The very success of the generic model in driving down prices, when taken to its extreme, threatens its ability to provide a consistent and reliable supply of essential medicines.
Section 7: The Future of Generics – Navigating New Frontiers and Headwinds
The generic drug industry stands at a strategic inflection point. The traditional business model, built on replicating simple small-molecule drugs and competing primarily on price, is being eroded by the very forces it unleashed. Relentless price pressure, global supply chain fragility, and rising regulatory complexity are forcing a fundamental evolution. In response, the industry is undergoing a strategic bifurcation, splitting into two increasingly distinct archetypes: the hyper-efficient commodity player and the science-driven “quasi-innovator.” The future will be defined by a company’s ability to choose its path and master the corresponding capabilities, from operational excellence at massive scale to cutting-edge scientific innovation.
The Rise of Complex Generics and Biosimilars
The primary strategy for companies seeking to escape the commoditization trap is to move up the value chain into products with higher barriers to entry, which promise more durable profitability. This “quasi-innovator” model is centered on two key areas: complex generics and biosimilars.
- Complex Generics: This category includes products that are difficult to develop due to a complex active ingredient, a complex formulation, a complex route of delivery, or a complex drug-device combination.5 Examples include long-acting injectables, metered-dose inhalers, transdermal patches, and ophthalmic emulsions. The scientific and regulatory hurdles for these products are significantly higher, which naturally limits the number of competitors and allows for more sustainable pricing.
- Biosimilars: Biologics are large, complex protein-based drugs manufactured in living systems, and they represent one of the fastest-growing and most expensive segments of the pharmaceutical market. A biosimilar is a biological product that is “highly similar” to an already-approved innovator biologic (the reference product) and has no clinically meaningful differences in terms of safety, purity, and potency.11 The development pathway for biosimilars, established in the U.S. by the Biologics Price Competition and Innovation Act (BPCIA) of 2010, is far more arduous and expensive than for traditional generics. Development can cost between $100 million and $300 million and take 6-9 years, compared to just $2-10 million for a small-molecule generic.43 Innovator biologics also enjoy a longer 12-year period of market exclusivity, and patent litigation follows a unique, highly structured process known as the “patent dance”.11 Despite these challenges, the biosimilar market is poised for explosive growth, with dozens of blockbuster biologics with combined sales in the hundreds of billions of dollars set to lose exclusivity by 2030.81
The Impact of Disruptive Technologies
Technology is no longer a peripheral support function but a core competitive differentiator, particularly for companies pursuing the specialty/quasi-innovator model.
- Artificial Intelligence (AI) and Machine Learning (ML): AI is revolutionizing the development process. ML algorithms can analyze vast datasets to predict API stability, optimize excipient selection in formulations, and design more efficient bioequivalence study protocols.84 In manufacturing, AI-powered computer vision can improve quality control by detecting defects in finished products.86 The FDA itself recognizes the growing role of AI and is working to develop a regulatory framework to support its use in drug development.88
- Advanced Manufacturing: Technologies like continuous manufacturing, where the production process runs nonstop in an integrated flow, are replacing traditional batch-based methods. This can dramatically improve efficiency, reduce the manufacturing footprint, and enhance product quality and consistency.43
3D printing is also emerging as a technology to create novel dosage forms with complex geometries or patient-specific dosing.43 - Blockchain: The integrity and security of the global pharmaceutical supply chain are paramount. Blockchain technology offers a decentralized, immutable digital ledger that can provide secure, transparent, end-to-end tracking of drugs from the manufacturer to the pharmacy.89 This enhances traceability, helps prevent counterfeit medicines from entering the supply chain, and simplifies compliance with regulations like the U.S. Drug Supply Chain Security Act (DSCSA).9
The Headwinds of Policy Change: The Inflation Reduction Act (IRA)
A major new variable shaping the future of the U.S. market is the Inflation Reduction Act of 2022. While primarily targeting brand-name drug prices, the IRA has significant downstream implications for the generic industry.76 By empowering Medicare to negotiate a “maximum fair price” for top-selling drugs nine years (for small molecules) or thirteen years (for biologics) after their approval, the IRA effectively lowers the brand price
before patent expiration.5 This shrinks the potential market size and revenue opportunity—the “pot of gold” at the end of the patent cliff—that has historically incentivized generic companies to undertake risky and expensive patent challenges. This could dampen the incentive for generic entry, particularly for small-molecule drugs, and alter the strategic calculations for product selection.
Concluding Thoughts: The Imperative to Evolve
The generic drug industry is at a crossroads. The forces of commoditization and global competition are unrelenting, making the old model of business unsustainable for all but the largest-scale players. Survival and prosperity in the coming decade will depend on a company’s ability to make a clear strategic choice and execute flawlessly. Whether the path is to become a low-cost, hyper-efficient producer of commodity generics or to evolve into a science-driven developer of complex, high-value specialty products, the fundamental principle remains the same. Success will belong to those who can most effectively harness information—from the intricacies of patent law and the complexities of global regulation to the frontiers of manufacturing technology—and transform it into a durable competitive advantage. In this new era, the ability to turn data into market-defining strategy, using every available tool, will be more critical than ever.
| Characteristic | Traditional Generics (Small Molecule) | Biosimilars (Large Molecule) | |
| Molecule | Simple, well-defined chemical structure. Identical copy is possible. | Large, complex protein structure. “Highly similar” copy, not identical. | |
| Development Cost | $2 million – $10 million | $100 million – $300 million | |
| Regulatory Pathway (U.S.) | Abbreviated New Drug Application (ANDA) | Biologics License Application (351(k) BLA) | |
| Innovator Exclusivity (U.S.) | 5 years (New Chemical Entity) | 12 years (First Licensure) | |
| Manufacturing Complexity | Relatively simple, well-established chemical synthesis. | Highly complex process in living cells; sensitive to process changes. | |
| Market Dynamics | Rapid uptake; intense price erosion (often >90%). | Slower uptake; less price erosion; requires physician education. | |
| Data synthesized from 11 |
Works cited
- Generic Medicines, accessed August 3, 2025, https://accessiblemeds.org/about/generic-medicines/
- Report: 2023 U.S. Generic and Biosimilar Medicines Savings Report, accessed August 3, 2025, https://accessiblemeds.org/resources/reports/2023-savings-report-2/
- Association for Accessible Medicines: Home, accessed August 3, 2025, https://accessiblemeds.org/
- 2024 U.S. Generic & Biosimilar Medicines Savings Report …, accessed August 3, 2025, https://accessiblemeds.org/resources/reports/2024-savings-report/
- The Global Generic Drug Market: Trends, Opportunities, and …, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/the-global-generic-drug-market-trends-opportunities-and-challenges/
- Generic Drugs Can Help Promote Health Equity – FDA, accessed August 3, 2025, https://www.fda.gov/media/173765/download
- Domestic pharma industry may face setback if US imposes tariffs, accessed August 3, 2025, https://timesofindia.indiatimes.com/business/india-business/domestic-pharma-industry-may-face-setback-if-us-imposes-tariffs/articleshow/123002989.cms
- Top 10 Challenges in Generic Drug Development – DrugPatentWatch, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/top-10-challenges-in-generic-drug-development/
- Streamlining the Generic Drug Supply Chain: Best Practices – DrugPatentWatch, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/streamlining-the-generic-drug-supply-chain-best-practices/
- 7 FAQs About Generic Drugs | Pfizer, accessed August 3, 2025, https://www.pfizer.com/news/articles/7_faqs_about_generic_drugs
- Understanding the Lifecycle of Generic Drugs: From Patent Cliffs to …, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/understanding-the-lifecycle-of-generic-drugs-from-development-to-market-impact/
- The Regulatory Pathway for Generic Drugs: A Strategic Guide to Market Entry and Competitive Advantage – DrugPatentWatch, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/the-regulatory-pathway-for-generic-drugs-explained/
- Hatch-Waxman Act | Practical Law, accessed August 3, 2025, https://uk.practicallaw.thomsonreuters.com/9-543-2565?transitionType=Default&contextData=(sc.Default)
- Drug Price Competition and Patent Term Restoration Act – Wikipedia, accessed August 3, 2025, https://en.wikipedia.org/wiki/Drug_Price_Competition_and_Patent_Term_Restoration_Act
- Hatch-Waxman 101 – Fish & Richardson, accessed August 3, 2025, https://www.fr.com/insights/thought-leadership/blogs/hatch-waxman-101-3/
- Abbreviated New Drug Applications (ANDA) Explained: A Quick-Guide – The FDA Group, accessed August 3, 2025, https://www.thefdagroup.com/blog/abbreviated-new-drug-applications-anda
- Abbreviated New Drug Application (ANDA) – FDA, accessed August 3, 2025, https://www.fda.gov/drugs/types-applications/abbreviated-new-drug-application-anda
- The ANDA Process: A Guide to FDA Submission & Approval – Excedr, accessed August 3, 2025, https://www.excedr.com/blog/what-is-abbreviated-new-drug-application
- FDA List of Authorized Generic Drugs, accessed August 3, 2025, https://www.fda.gov/drugs/abbreviated-new-drug-application-anda/fda-list-authorized-generic-drugs
- Generic Drugs: Questions & Answers | FDA, accessed August 3, 2025, https://www.fda.gov/drugs/frequently-asked-questions-popular-topics/generic-drugs-questions-answers
- The Hatch-Waxman Act: A Primer – EveryCRSReport.com, accessed August 3, 2025, https://www.everycrsreport.com/reports/R44643.html
- Hatch-Waxman Related Provisions of the Medicare Prescription Drug Bills (H.R. 1 and S. 1) – UM Carey Law, accessed August 3, 2025, https://www2.law.umaryland.edu/marshall/crsreports/crsdocuments/RL32003.pdf
- Small Business Assistance | 180-Day Generic Drug Exclusivity – FDA, accessed August 3, 2025, https://www.fda.gov/drugs/cder-small-business-industry-assistance-sbia/small-business-assistance-180-day-generic-drug-exclusivity
- What Every Pharma Executive Needs to Know About Paragraph IV …, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/what-every-pharma-executive-needs-to-know-about-paragraph-iv-challenges/
- The Growing Importance of Generic Drug Development for Emerging Markets, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/the-growing-importance-of-generic-drug-development-for-emerging-markets/
- The End of Exclusivity: Navigating the Drug Patent Cliff for Competitive Advantage – DrugPatentWatch – Transform Data into Market Domination, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/the-impact-of-drug-patent-expiration-financial-implications-lifecycle-strategies-and-market-transformations/
- DrugPatentWatch | Software Reviews & Alternatives – Crozdesk, accessed August 3, 2025, https://crozdesk.com/software/drugpatentwatch
- Cracking the Code: Using Drug Patents to Reveal Competitor Formulation Strategies, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/cracking-the-code-using-drug-patents-to-reveal-competitor-formulation-strategies/
- Generic Drug Entry Timeline: Predicting Market Dynamics After Patent Loss, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/generic-drug-entry-timeline-predicting-market-dynamics-after-patent-loss/
- Drug Competition Series – Analysis of New Generic Markets Effect of Market Entry on Generic Drug Prices – HHS ASPE, accessed August 3, 2025, https://aspe.hhs.gov/sites/default/files/documents/510e964dc7b7f00763a7f8a1dbc5ae7b/aspe-ib-generic-drugs-competition.pdf
- Quality Considerations for Developing Complex Generics – FDA, accessed August 3, 2025, https://www.fda.gov/media/168931/download
- Bioequivalence Studies & Complex Generics – BioPharma Services, accessed August 3, 2025, https://www.biopharmaservices.com/blog/bioequivalence-studies-and-complex-generics/
- Remarks to FDA employees on Generic Drug Science Day – 11/28/2017, accessed August 3, 2025, https://www.fda.gov/news-events/speeches-fda-officials/remarks-fda-employees-generic-drug-science-day-11282017
- The 180-Day Rule Supports Generic Competition. Here’s How …, accessed August 3, 2025, https://accessiblemeds.org/resources/blog/180-day-rule-supports-generic-competition-heres-how/
- Development of Generic Drug Products by Pharmaceutical Industries Considering Regulatory Aspects: A Review – Scientific Research Publishing, accessed August 3, 2025, https://www.scirp.org/journal/paperinformation?paperid=112370
- Generic Product Development and Reverse Engineering of Reference- Listed Drug Product – Impactfactor, accessed August 3, 2025, http://impactfactor.org/PDF/IJDDT/15/IJDDT,Vol15,Issue1,Article48.pdf
- en.wikipedia.org, accessed August 3, 2025, https://en.wikipedia.org/wiki/Generic_drug#:~:text=A%20generic%20drug%20must%20contain,to%20pharmacokinetic%20and%20pharmacodynamic%20properties.
- Facts About Generic Drugs – FDA, accessed August 3, 2025, https://www.fda.gov/media/83670/download
- Generic Development of Topical Dermatologic Products: Formulation Development, Process Development, and Testing of Topical Dermatologic Products – PMC – PubMed Central, accessed August 3, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC3535108/
- FDA Critical Path Initiatives: Opportunities for Generic Drug Development – PMC, accessed August 3, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC2751455/
- Pharmaceutical Formulation Development | Malvern Panalytical, accessed August 3, 2025, https://www.malvernpanalytical.com/en/industries/pharmaceuticals/pharmaceutical-formulation-development
- Precision and Reproducibility in Generic Drug Reverse Engineering – DrugPatentWatch, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/precision-and-reproducibility-in-generic-drug-reverse-engineering/
- Innovations in Generic Drug Manufacturing and Formulation: Transforming Products and Reducing Costs – DrugPatentWatch, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/innovations-in-generic-drug-manufacturing-and-formulation-transforming-products-and-reducing-costs/
- How Generic Drugs Are Developed : Pharmaceutical process – ZIM Labs, accessed August 3, 2025, https://www.zimlab.in/blog-posts/how-generic-drugs-are-developed-a-deep-dive-into-the-pharmaceutical-process
- Analytical Method Development and Validation: Cost-Effective Approaches for Generic Drug Testing – DrugPatentWatch, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/analytical-method-development-and-validation-cost-effective-approaches-for-generic-drug-testing/
- Regulatory Affairs: What is the New FDA Guidance on Q14 Analytical Procedure Development? | Contract Pharma, accessed August 3, 2025, https://www.contractpharma.com/exclusives/regulatory-affairs-what-is-the-new-fda-guidance-on-q14-analytical-procedure-development/
- Are We Embracing FDA’s Messages? – Contract Pharma, accessed August 3, 2025, https://www.contractpharma.com/are-we-embracing-fdas-messages/
- Good manufacturing practice | European Medicines Agency (EMA), accessed August 3, 2025, https://www.ema.europa.eu/en/human-regulatory-overview/research-development/compliance-research-development/good-manufacturing-practice
- Good Manufacturing Practices (GMP) standards – World Health Organization (WHO), accessed August 3, 2025, https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/norms-and-standards/gmp
- Trump tariffs to push up US drug prices, won’t change India’s pharma growth playbook: Pharmexcil, accessed August 3, 2025, https://economictimes.indiatimes.com/small-biz/trade/exports/insights/trump-tariffs-to-push-up-us-drug-prices-wont-change-indias-pharma-growth-playbook-pharmexcil/articleshow/123041531.cms
- Abbreviated New Drug Application (ANDA) Forms and Submission Requirements – FDA, accessed August 3, 2025, https://www.fda.gov/drugs/abbreviated-new-drug-application-anda/abbreviated-new-drug-application-anda-forms-and-submission-requirements
- Abbreviated New Drug Application (ANDA) Approval Process – FDA, accessed August 3, 2025, https://www.fda.gov/media/166141/download
- Bioequivalence and Interchangeability of Generic Drugs – Merck Manuals, accessed August 3, 2025, https://www.merckmanuals.com/home/drugs/brand-name-and-generic-drugs/bioequivalence-and-interchangeability-of-generic-drugs
- Bioavailability and Bioequivalence in Drug Development – PMC, accessed August 3, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC4157693/
- Bioequivalence Studies and Their Role in Drug Development – Biostatistics.ca, accessed August 3, 2025, https://www.biostatistics.ca/bioequivalence-studies-and-their-role-in-drug-development/
- A Comparative Overview of Generic Drug Regulation in US, Europe, Australia and India, accessed August 3, 2025, https://www.ijdra.com/index.php/journal/article/download/747/396
- Bioequivalence Studies for Generic Drug Development | FDA, accessed August 3, 2025, https://www.fda.gov/media/166152/download
- An adaptive trial design for testing the bioequivalence of generics of highly variable drugs, accessed August 3, 2025, https://www.fda.gov/drugs/regulatory-science-action/adaptive-trial-design-testing-bioequivalence-generics-highly-variable-drugs
- Bioequivalence – FDA, accessed August 3, 2025, https://www.fda.gov/animal-veterinary/abbreviated-new-animal-drug-applications/bioequivalence
- Regulatory pathways in the European Union – PMC – PubMed Central, accessed August 3, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC3149704/
- Generic Drug Market Entry in Europe: Why a Tailored Approach Is Best – DrugPatentWatch, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/generic-drug-market-entry-in-europe-why-a-tailored-approach-is-best/
- Generic and hybrid applications | European Medicines Agency (EMA), accessed August 3, 2025, https://www.ema.europa.eu/en/human-regulatory-overview/marketing-authorisation/generic-hybrid-medicines/generic-hybrid-applications
- Authorisation of medicines – EMA – European Union, accessed August 3, 2025, https://www.ema.europa.eu/en/about-us/what-we-do/authorisation-medicines
- Key steps and considerations of the EU centralised procedure – TOPRA, accessed August 3, 2025, https://www.topra.org/TOPRA/TOPRA_MEMBER/PDFs/TOPRA-RR-CPD-JULAUG2019.PDF
- How Generic Drugs are Registered in Europe, United Kingdom, Australia and New Zealand? – International Journal of Pharmaceutical Investigation, accessed August 3, 2025, https://jpionline.org/storage/2023/07/IntJPharmInvestigation-13-3-440.pdf
- Decentralised procedure | European Medicines Agency (EMA), accessed August 3, 2025, https://www.ema.europa.eu/en/glossary-terms/decentralised-procedure
- EGA wants better Centralised Procedure and Decentralised Procedure for generics authorisation – Generics and Biosimilars Initiative, accessed August 3, 2025, https://gabionline.net/generics/general/EGA-wants-better-Centralised-Procedure-and-Decentralised-Procedure-for-generics-authorisation
- Authorized Generics: What You Need to Know – GoodRx, accessed August 3, 2025, https://www.goodrx.com/drugs/medication-basics/what-are-authorized-generics
- Authorized Generics In The US: Prevalence, Characteristics, And Timing, 2010–19, accessed August 3, 2025, https://www.healthaffairs.org/doi/10.1377/hlthaff.2022.01677
- Pharmaceutical Patents, Paragraph IV, and Pay-for-Delay – Digital Commons @ American University Washington College of, accessed August 3, 2025, https://digitalcommons.wcl.american.edu/cgi/viewcontent.cgi?article=1024&context=ipbrief
- The High Cost of Waiting: An Analysis of Pay-for-Delay Agreements in the Pharmaceutical Industry – The St Andrews Economist, accessed August 3, 2025, https://standrewseconomist.com/2024/03/18/the-high-cost-of-waiting-an-analysis-of-pay-for-delay-agreements-in-the-pharmaceutical-industry/
- Pay for Delay | Federal Trade Commission, accessed August 3, 2025, https://www.ftc.gov/news-events/topics/competition-enforcement/pay-delay
- Pay for Delay: Historic and Future Costs of Delayed Generic Entry – ARC, accessed August 3, 2025, https://web.aresearch.com/wp-content/uploads/2025/04/Pay-for-Delay-Brief-2025.4.8.pdf
- The Impact of Patent Expirations on Generic Drug Market Entry – PatentPC, accessed August 3, 2025, https://patentpc.com/blog/the-impact-of-patent-expirations-on-generic-drug-market-entry
- Effects of Generic Entry on Market Shares and Prices of Originator Drugs: Evidence from Chinese Pharmaceutical Market – PubMed Central, accessed August 3, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC12209137/
- Decoding Drug Pricing Models: A Strategic Guide to Market Domination – DrugPatentWatch, accessed August 3, 2025, https://www.drugpatentwatch.com/blog/decoding-drug-pricing-models-a-strategic-guide-to-market-domination/
- 2023 Report | Association for Accessible Medicines, accessed August 3, 2025, https://accessiblemeds.org/resources/blog/2023-savings-report/
- Report: 2022 U.S. Generic and Biosimilar Medicines Savings Report, accessed August 3, 2025, https://accessiblemeds.org/resources/reports/2022-savings-report/
- The impact of drug pricing and reimbursement on the pharmaceutical industry, accessed August 3, 2025, https://www.clinicaltrialsarena.com/sponsored/the-impact-of-drug-pricing-and-reimbursement-on-the-pharmaceutical-industry/
- Consumers are now actively seeking generic substitutes for branded medicines: Sujit Paul, Zota Healthcare, accessed August 3, 2025, https://economictimes.indiatimes.com/small-biz/entrepreneurship/consumers-are-now-actively-seeking-generic-substitutes-for-branded-medicines-sujit-paul-zota-healthcare/articleshow/122987675.cms
- Trends in Biosimilars – Clinical Research News, accessed August 3, 2025, https://www.clinicalresearchnewsonline.com/news/2024/11/22/trends-in-biosimilars
- FDA Approval Stats: Trends in First Generics, Biosimilars, and More – GoodRx, accessed August 3, 2025, https://www.goodrx.com/drugs/news/recent-fda-approval-statistics
- The Global Market For Generics Biosimilars Through 2032 – Outsourced Pharma, accessed August 3, 2025, https://www.outsourcedpharma.com/doc/the-global-market-for-generics-biosimilars-through-2032-0001
- Harnessing Artificial Intelligence in Generic Formulation Development and Life Cycle Management – A Comprehensive Review, accessed August 3, 2025, https://healthinformaticsjournal.al-makkipublisher.com/index.php/hij/article/view/45
- Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design – PMC, accessed August 3, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10385763/
- The Impact of Artificial Intelligence on Drug Development and Formulation – YMER, accessed August 3, 2025, https://ymerdigital.com/uploads/YMER240268.pdf
- Digital Transformation Can Accelerate Generic Drug Development, Optimizing Manufacturing Processes. – GeneOnline News, accessed August 3, 2025, https://www.geneonline.com/digital-transformation-can-accelerate-generic-drug-development-optimizing-manufacturing-processes/
- Artificial Intelligence for Drug Development – FDA, accessed August 3, 2025, https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/artificial-intelligence-drug-development
- Blockchain- How to Integrate into Pharmaceutical Industry? – Venus Remedies, accessed August 3, 2025, https://venusremedies.com/blog/blockchain
- Disrupting Pharma Industry with Blockchain – Nitor Infotech, accessed August 3, 2025, https://www.nitorinfotech.com/blog/disrupting-pharma-industry-with-blockchain/
- Blockchain: A Game-Changer for Pharma Supply Chains – Binariks, accessed August 3, 2025, https://binariks.com/blog/blockchain-pharma-supply-chain/
- How Digital Transformation is Accelerating Drug Development – PCI Pharma Services, accessed August 3, 2025, https://pci.com/resources/lab-to-market/