32 Drugs Facing NCE-1 / Abbreviated New Drug Application acceptance dates in 2025 - 2026
Loss of Exclusivity / End of Market Exclusivity Period dates
The content of this page is licensed under a Creative Commons Attribution 4.0 International License.

Friedman, Yali, "32 Drugs Facing NCE-1 / Abbreviated New Drug Application acceptance dates in 2025 - 2026" DrugPatentWatch.com thinkBiotech, 2025 www.drugpatentwatch.com/p/nce-1/.
Media collateral
These NCE-1 dates indicate the first opportunity for generic drug companies to file Abbreviated New Drug Applications (ANDAs) for generic entry into branded drug markets. Generic launch is dependent on many factors, including FDA approval and patents. This information is provided as a rough estimate of generic application, and does not indicate when generics will launch. For more information see the complete DrugPatentWatch database.
When can drug patent challenges be filed against CIBINQO?
Generic name: abrocitinib
NCE-1 Date: January 2026
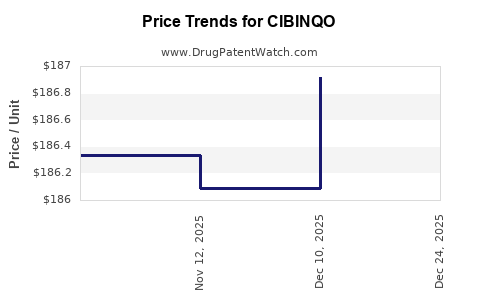
This drug has sixty-five patent family members in forty-four countries.
See drug price trends for CIBINQO.
The generic ingredient in CIBINQO is abrocitinib. Additional details are available on the abrocitinib profile page.
When can drug patent challenges be filed against PYRUKYND?
Generic name: mitapivat sulfate
NCE-1 Date: February 2026
PYRUKYND is a drug marketed by Agios Pharms Inc. There are nine patents protecting this drug.
This drug has one hundred and ninety-one patent family members in forty-six countries.
See drug price trends for PYRUKYND.
The generic ingredient in PYRUKYND is mitapivat sulfate. Additional details are available on the mitapivat sulfate profile page.
When can drug patent challenges be filed against VONJO?
Generic name: pacritinib citrate
NCE-1 Date: February 2026
VONJO is a drug marketed by Sobi. There are three patents protecting this drug.
This drug has seventy-eight patent family members in twenty-eight countries.
See drug price trends for VONJO.
The generic ingredient in VONJO is pacritinib citrate. Additional details are available on the pacritinib citrate profile page.
When can drug patent challenges be filed against PLUVICTO?
Generic name: lutetium lu-177 vipivotide tetraxetan
NCE-1 Date: March 2026
PLUVICTO is a drug marketed by Novartis. There are five patents protecting this drug.
This drug has one hundred and thirty-two patent family members in thirty-nine countries.
See drug price trends for PLUVICTO.
The generic ingredient in PLUVICTO is lutetium lu-177 vipivotide tetraxetan. There are four drug master file entries for this API. Additional details are available on the lutetium lu-177 vipivotide tetraxetan profile page.
When can drug patent challenges be filed against QUVIVIQ?
Generic name: daridorexant hydrochloride
NCE-1 Date: April 2026
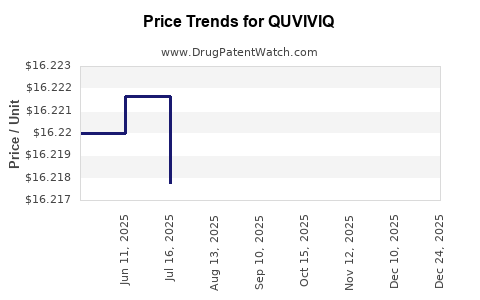
This drug has eighty-eight patent family members in thirty-five countries.
See drug price trends for QUVIVIQ.
The generic ingredient in QUVIVIQ is daridorexant hydrochloride. Additional details are available on the daridorexant hydrochloride profile page.
When can drug patent challenges be filed against VIVJOA?
Generic name: oteseconazole
NCE-1 Date: April 2026
VIVJOA is a drug marketed by Mycovia Pharms. There are five patents protecting this drug.
This drug has sixty-four patent family members in twenty-two countries.
See drug price trends for VIVJOA.
The generic ingredient in VIVJOA is oteseconazole. Additional details are available on the oteseconazole profile page.
When can drug patent challenges be filed against CAMZYOS?
Generic name: mavacamten
NCE-1 Date: April 2026
CAMZYOS is a drug marketed by Bristol. There are two patents protecting this drug.
This drug has sixty-seven patent family members in thirty-nine countries.
See drug price trends for CAMZYOS.
The generic ingredient in CAMZYOS is mavacamten. Additional details are available on the mavacamten profile page.
When can drug patent challenges be filed against VOQUEZNA?
Generic name: vonoprazan fumarate
NCE-1 Date: May 2026
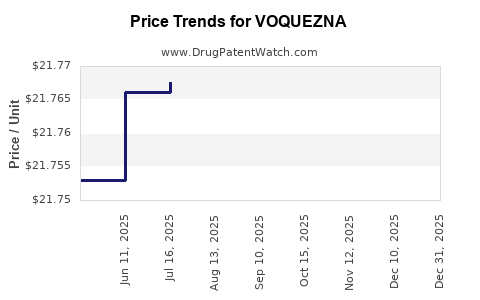
This drug has one hundred and three patent family members in forty-three countries.
See drug price trends for VOQUEZNA.
The generic ingredient in VOQUEZNA is vonoprazan fumarate. There is one drug master file entry for this API. Additional details are available on the vonoprazan fumarate profile page.
When can drug patent challenges be filed against VOQUEZNA TRIPLE PAK?
Generic name: amoxicillin; clarithromycin; vonoprazan fumarate
NCE-1 Date: May 2026
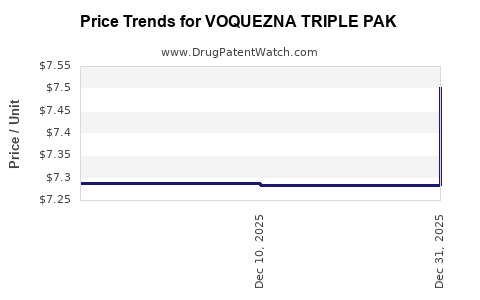
This drug has one hundred and three patent family members in forty-three countries.
See drug price trends for VOQUEZNA TRIPLE PAK.
The generic ingredient in VOQUEZNA TRIPLE PAK is amoxicillin; clarithromycin; vonoprazan fumarate. There are forty-six drug master file entries for this API. Additional details are available on the amoxicillin; clarithromycin; vonoprazan fumarate profile page.
When can drug patent challenges be filed against VOQUEZNA DUAL PAK?
Generic name: amoxicillin; vonoprazan fumarate
NCE-1 Date: May 2026
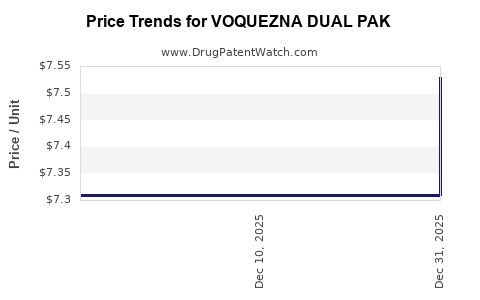
This drug has one hundred and three patent family members in forty-three countries.
See drug price trends for VOQUEZNA DUAL PAK.
The generic ingredient in VOQUEZNA DUAL PAK is amoxicillin; vonoprazan fumarate. There are forty-six drug master file entries for this API. Additional details are available on the amoxicillin; vonoprazan fumarate profile page.
When can drug patent challenges be filed against MOUNJARO?
Generic name: tirzepatide
NCE-1 Date: May 2026
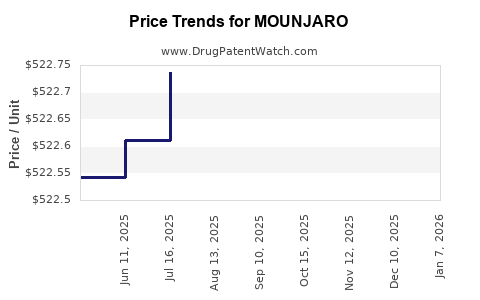
This drug has one hundred and ninety-three patent family members in forty-six countries. There has been litigation on patents covering MOUNJARO
See drug price trends for MOUNJARO.
The generic ingredient in MOUNJARO is tirzepatide. Additional details are available on the tirzepatide profile page.
When can drug patent challenges be filed against MOUNJARO (AUTOINJECTOR)?
Generic name: tirzepatide
NCE-1 Date: May 2026
MOUNJARO (AUTOINJECTOR) is a drug marketed by Eli Lilly And Co. There are four patents protecting this drug.
This drug has one hundred and ninety-three patent family members in forty-six countries.
The generic ingredient in MOUNJARO (AUTOINJECTOR) is tirzepatide. Additional details are available on the tirzepatide profile page.
When can drug patent challenges be filed against ZEPBOUND?
Generic name: tirzepatide
NCE-1 Date: May 2026
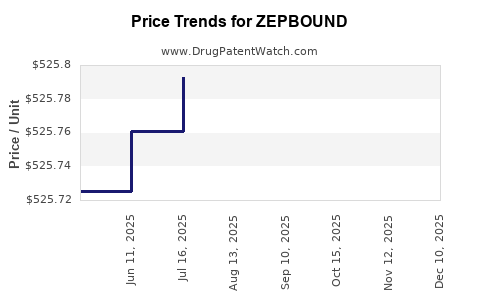
This drug has one hundred and fifty-eight patent family members in forty-six countries. There has been litigation on patents covering ZEPBOUND
See drug price trends for ZEPBOUND.
The generic ingredient in ZEPBOUND is tirzepatide. Additional details are available on the tirzepatide profile page.
When can drug patent challenges be filed against ZEPBOUND (AUTOINJECTOR)?
Generic name: tirzepatide
NCE-1 Date: May 2026
ZEPBOUND (AUTOINJECTOR) is a drug marketed by Eli Lilly And Co. There are four patents protecting this drug.
This drug has one hundred and fifty-eight patent family members in forty-six countries.
The generic ingredient in ZEPBOUND (AUTOINJECTOR) is tirzepatide. Additional details are available on the tirzepatide profile page.
When can drug patent challenges be filed against VTAMA?
Generic name: tapinarof
NCE-1 Date: May 2026
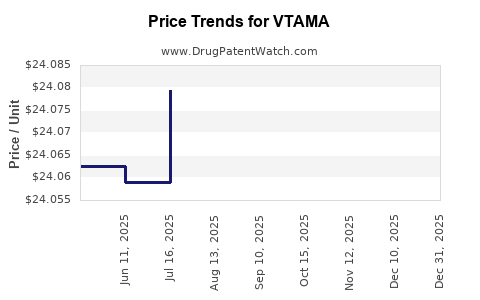
This drug has eighty patent family members in thirty-nine countries. There has been litigation on patents covering VTAMA
See drug price trends for VTAMA.
The generic ingredient in VTAMA is tapinarof. Additional details are available on the tapinarof profile page.
When can drug patent challenges be filed against ZTALMY?
Generic name: ganaxolone
NCE-1 Date: June 2026
ZTALMY is a drug marketed by Marinus. There are eleven patents protecting this drug.
This drug has forty-eight patent family members in sixteen countries. There has been litigation on patents covering ZTALMY
See drug price trends for ZTALMY.
The generic ingredient in ZTALMY is ganaxolone. Additional details are available on the ganaxolone profile page.
When can drug patent challenges be filed against AMVUTTRA?
Generic name: vutrisiran sodium
NCE-1 Date: June 2026
AMVUTTRA is a drug marketed by Alnylam Pharms Inc. There are thirteen patents protecting this drug.
This drug has two hundred and fifty-five patent family members in forty-eight countries. There has been litigation on patents covering AMVUTTRA
See drug price trends for AMVUTTRA.
The generic ingredient in AMVUTTRA is vutrisiran sodium. Additional details are available on the vutrisiran sodium profile page.
When can drug patent challenges be filed against BLUDIGO?
Generic name: indigotindisulfonate sodium
NCE-1 Date: July 2026
BLUDIGO is a drug marketed by Provepharm Sas. There are three patents protecting this drug.
This drug has two patent family members in two countries.
See drug price trends for BLUDIGO.
The generic ingredient in BLUDIGO is indigotindisulfonate sodium. There are two drug master file entries for this API. Additional details are available on the indigotindisulfonate sodium profile page.
When can drug patent challenges be filed against SOTYKTU?
Generic name: deucravacitinib
NCE-1 Date: September 2026
SOTYKTU is a drug marketed by Bristol. There are three patents protecting this drug.
This drug has seventy patent family members in thirty-eight countries.
See drug price trends for SOTYKTU.
The generic ingredient in SOTYKTU is deucravacitinib. Additional details are available on the deucravacitinib profile page.
When can drug patent challenges be filed against TERLIVAZ?
Generic name: terlipressin acetate
NCE-1 Date: September 2026
TERLIVAZ is a drug marketed by Mallinckrodt Ireland. There is one patent protecting this drug.
This drug has thirty-six patent family members in seventeen countries.
See drug price trends for TERLIVAZ.
The generic ingredient in TERLIVAZ is terlipressin acetate. There are two drug master file entries for this API. Additional details are available on the terlipressin acetate profile page.
When can drug patent challenges be filed against ELUCIREM?
Generic name: gadopiclenol
NCE-1 Date: September 2026
ELUCIREM is a drug marketed by Guerbet. There are three patents protecting this drug.
This drug has one hundred and seven patent family members in twenty-eight countries.
See drug price trends for ELUCIREM.
The generic ingredient in ELUCIREM is gadopiclenol. Additional details are available on the gadopiclenol profile page.
When can drug patent challenges be filed against OMLONTI?
Generic name: omidenepag isopropyl
NCE-1 Date: September 2026
OMLONTI is a drug marketed by Ocuvex Therap. There are thirteen patents protecting this drug.
This drug has one hundred and thirty-four patent family members in thirty-two countries.
The generic ingredient in OMLONTI is omidenepag isopropyl. Additional details are available on the omidenepag isopropyl profile page.
When can drug patent challenges be filed against RELYVRIO?
Generic name: sodium phenylbutyrate; taurursodiol
NCE-1 Date: September 2026
RELYVRIO is a drug marketed by Amylyx. There are five patents protecting this drug.
This drug has sixty-three patent family members in twenty-five countries.
See drug price trends for RELYVRIO.
The generic ingredient in RELYVRIO is sodium phenylbutyrate; taurursodiol. There are one thousand four hundred and seventy-two drug master file entries for this API. Additional details are available on the sodium phenylbutyrate; taurursodiol profile page.
When can drug patent challenges be filed against LYTGOBI?
Generic name: futibatinib
NCE-1 Date: September 2026
LYTGOBI is a drug marketed by Taiho Oncology. There are three patents protecting this drug.
This drug has seventy-nine patent family members in twenty-five countries.
See drug price trends for LYTGOBI.
The generic ingredient in LYTGOBI is futibatinib. Additional details are available on the futibatinib profile page.
When can drug patent challenges be filed against REZLIDHIA?
Generic name: olutasidenib
NCE-1 Date: December 2026
REZLIDHIA is a drug marketed by Rigel Pharms. There are fourteen patents protecting this drug.
This drug has one hundred and ten patent family members in thirty-eight countries. There has been litigation on patents covering REZLIDHIA
See drug price trends for REZLIDHIA.
The generic ingredient in REZLIDHIA is olutasidenib. Additional details are available on the olutasidenib profile page.
When can drug patent challenges be filed against KRAZATI?
Generic name: adagrasib
NCE-1 Date: December 2026
KRAZATI is a drug marketed by Bristol. There are four patents protecting this drug.
This drug has eighty-seven patent family members in thirty-four countries.
See drug price trends for KRAZATI.
The generic ingredient in KRAZATI is adagrasib. Additional details are available on the adagrasib profile page.
When can drug patent challenges be filed against SUNLENCA?
Generic name: lenacapavir sodium
NCE-1 Date: December 2026
SUNLENCA is a drug marketed by Gilead Sciences Inc. There are five patents protecting this drug.
This drug has two hundred and ten patent family members in forty-seven countries.
See drug price trends for SUNLENCA.
The generic ingredient in SUNLENCA is lenacapavir sodium. Additional details are available on the lenacapavir sodium profile page.
When can drug patent challenges be filed against YEZTUGO?
Generic name: lenacapavir sodium
NCE-1 Date: December 2026
YEZTUGO is a drug marketed by Gilead Sciences Inc. There are four patents protecting this drug.
This drug has two hundred patent family members in forty-seven countries.
The generic ingredient in YEZTUGO is lenacapavir sodium. Additional details are available on the lenacapavir sodium profile page.
When can drug patent challenges be filed against XENOVIEW?
Generic name: xenon xe-129 hyperpolarized
NCE-1 Date: December 2026
XENOVIEW is a drug marketed by Polarean. There are two patents protecting this drug.
This drug has six patent family members in six countries.
The generic ingredient in XENOVIEW is xenon xe-129 hyperpolarized. There are twelve drug master file entries for this API. Additional details are available on the xenon xe-129 hyperpolarized profile page.
When can drug patent challenges be filed against BRENZAVVY?
Generic name: bexagliflozin
NCE-1 Date: January 2027
BRENZAVVY is a drug marketed by Theracosbio. There are six patents protecting this drug.
This drug has seventy-three patent family members in thirty-four countries. There has been litigation on patents covering BRENZAVVY
The generic ingredient in BRENZAVVY is bexagliflozin. Additional details are available on the bexagliflozin profile page.
When can drug patent challenges be filed against ORSERDU?
Generic name: elacestrant hydrochloride
NCE-1 Date: January 2027
ORSERDU is a drug marketed by Stemline Therap. There are eight patents protecting this drug.
This drug has one hundred and sixty-two patent family members in twenty-nine countries. There has been litigation on patents covering ORSERDU
See drug price trends for ORSERDU.
The generic ingredient in ORSERDU is elacestrant hydrochloride. Additional details are available on the elacestrant hydrochloride profile page.
When can drug patent challenges be filed against JAYPIRCA?
Generic name: pirtobrutinib
NCE-1 Date: January 2027
JAYPIRCA is a drug marketed by Loxo Oncol. There are seven patents protecting this drug.
This drug has one hundred and four patent family members in thirty-nine countries.
See drug price trends for JAYPIRCA.
The generic ingredient in JAYPIRCA is pirtobrutinib. Additional details are available on the pirtobrutinib profile page.

