ZEPBOUND Drug Patent Profile
✉ Email this page to a colleague
When do Zepbound patents expire, and when can generic versions of Zepbound launch?
Zepbound is a drug marketed by Eli Lilly And Co and is included in one NDA. There are four patents protecting this drug.
This drug has one hundred and fifty-eight patent family members in forty-six countries.
The generic ingredient in ZEPBOUND is tirzepatide. One supplier is listed for this compound. Additional details are available on the tirzepatide profile page.
DrugPatentWatch® Generic Entry Outlook for Zepbound
Zepbound will be eligible for patent challenges on May 13, 2026. This date may extended up to six months if a pediatric exclusivity extension is applied to the drug's patents.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be January 5, 2036. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ZEPBOUND?
- What are the global sales for ZEPBOUND?
- What is Average Wholesale Price for ZEPBOUND?
Summary for ZEPBOUND
| International Patents: | 158 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 4 |
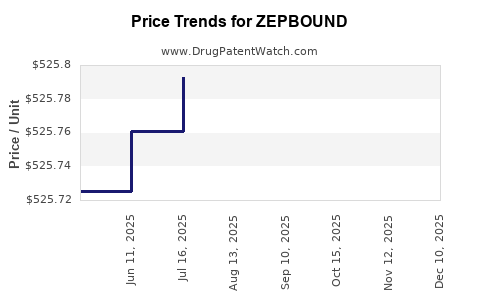
| Drug Prices: | Drug price information for ZEPBOUND |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ZEPBOUND |
| What excipients (inactive ingredients) are in ZEPBOUND? | ZEPBOUND excipients list |
| DailyMed Link: | ZEPBOUND at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ZEPBOUND
Generic Entry Date for ZEPBOUND*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;SUBCUTANEOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ZEPBOUND
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| The University of Texas Medical Branch, Galveston | PHASE2 |
| Scripps Translational Science Institute | PHASE2 |
| Schmidt Initiative for Long COVID | PHASE2 |
Pharmacology for ZEPBOUND
US Patents and Regulatory Information for ZEPBOUND
ZEPBOUND is protected by five US patents and three FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ZEPBOUND is ⤷ Get Started Free.
This potential generic entry date is based on patent ⤷ Get Started Free.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eli Lilly And Co | ZEPBOUND (AUTOINJECTOR) | tirzepatide | SOLUTION;SUBCUTANEOUS | 217806-003 | Nov 8, 2023 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Eli Lilly And Co | ZEPBOUND (AUTOINJECTOR) | tirzepatide | SOLUTION;SUBCUTANEOUS | 217806-006 | Nov 8, 2023 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Eli Lilly And Co | ZEPBOUND | tirzepatide | SOLUTION;SUBCUTANEOUS | 217806-012 | Mar 28, 2024 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Eli Lilly And Co | ZEPBOUND | tirzepatide | SOLUTION;SUBCUTANEOUS | 217806-010 | Mar 28, 2024 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Eli Lilly And Co | ZEPBOUND (AUTOINJECTOR) | tirzepatide | SOLUTION;SUBCUTANEOUS | 217806-001 | Nov 8, 2023 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for ZEPBOUND
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Eli Lilly Nederland B.V. | Mounjaro | tirzepatide | EMEA/H/C/005620Mounjaro is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise- as monotherapy when metformin is considered inappropriate due to intolerance or contraindications- in addition to other medicinal products for the treatment of diabetes.For study results with respect to combinations, effects on glycaemic control and the populations studied, see sections 4.4, 4.5 and 5.1. | Authorised | no | no | no | 2022-09-15 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ZEPBOUND
When does loss-of-exclusivity occur for ZEPBOUND?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 3242
Patent: COMPUESTOS CO-AGONISTAS DE GIP Y GLP-1
Estimated Expiration: ⤷ Get Started Free
Patent: 1857
Patent: COMPUESTOS CO-AGONISTAS DE GIP Y GLP-1
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 16205435
Patent: GIP and GLP-1 co-agonist compounds
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2017010596
Patent: compostos coagonistas de gip e glp-1
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 73352
Patent: COMPOSES CO-AGONISTES DE GIP ET DE GLP-1 (GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 17001760
Patent: Compuestos co-agonistas de gip y glp-1
Estimated Expiration: ⤷ Get Started Free
China
Patent: 7207576
Patent: GIP和GLP‑1共激动剂化合物 (GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 2608377
Patent: GIP和GLP-1共激动剂化合物 (GIP and GLP-1 co-agonist compounds)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 17006737
Patent: Análogos de gip y glp-1 en donde la lisina (k) en la posición 20 está químicamente modificada a través de la conjugación al grupo épsilon-amino de la cadena lateral k con ([2-(2-amino-etoxi)-etoxi]-acetil)2-(γglu)a-co-(ch2)b-co2h activos como agonistas de receptores gip y gip-1
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 170310
Patent: COMPUESTOS CO-AGONISTAS DE GIP Y GLP-1
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0191614
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 22028
Estimated Expiration: ⤷ Get Started Free
Patent: 23003
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 42887
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 017000153
Patent: COMPUESTOS CO-AGONISTAS DE GIP Y GLP-1
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 17043648
Patent: COMPUESTOS CO-AGONISTAS DE GIP Y GLP-1
Estimated Expiration: ⤷ Get Started Free
El Salvador
Patent: 17005453
Patent: COMPUESTOS CO-AGONISTAS DE GIP Y GLP-1
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 1591
Patent: СОЕДИНЕНИЯ-КОАГОНИСТЫ ГИП И ГПП-1 (GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 5055
Patent: СОЕДИНЕНИЯ-КОАГОНИСТЫ ГИП И ГПП-1 (GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 1791281
Patent: СОЕДИНЕНИЯ-КОАГОНИСТЫ ГИП И ГПП-1
Estimated Expiration: ⤷ Get Started Free
Patent: 1892057
Patent: СОЕДИНЕНИЯ-КОАГОНИСТЫ ГИП И ГПП-1
Estimated Expiration: ⤷ Get Started Free
Patent: 2090392
Patent: СОЕДИНЕНИЯ-КОАГОНИСТЫ ГИП И ГПП-1
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 42887
Patent: COMPOSÉS CO-AGONISTES DE GIP ET DE GLP-1 (GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 97662
Patent: COMPOSÉS CO-AGONISTES GIP ET GLP-1 (GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Finland
Patent: 0230005
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1006
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 45860
Estimated Expiration: ⤷ Get Started Free
Patent: 300006
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 2499
Patent: תרכובות קו-אגוניסטיות ל- gip ו- glp-1 (Gip and glp-1 co-agonist compounds)
Estimated Expiration: ⤷ Get Started Free
Patent: 6492
Patent: תרכובות קו-אגוניסטיות ל- gip ו- glp-1 (Gip and glp-1 co-agonist compounds)
Estimated Expiration: ⤷ Get Started Free
Patent: 1545
Patent: תרכובות קו-אגוניסטיות ל- gip ו- glp-1 (Gip and glp-1 co-agonist compounds)
Estimated Expiration: ⤷ Get Started Free
Patent: 0236
Patent: תרכובות קו-אגוניסטיות ל- gip ו- glp-1 (Gip and glp-1 co-agonist compounds)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 19534
Estimated Expiration: ⤷ Get Started Free
Patent: 45766
Estimated Expiration: ⤷ Get Started Free
Patent: 54867
Estimated Expiration: ⤷ Get Started Free
Patent: 17507124
Patent: GIPおよびGLP−1コアゴニスト化合物
Estimated Expiration: ⤷ Get Started Free
Patent: 18052933
Patent: GIPおよびGLP−1コアゴニスト化合物 (GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 19203000
Patent: GIPおよびGLP−1コアゴニスト化合物 (GIP AND GLP-1 CO-AGONIST COMPOUND)
Estimated Expiration: ⤷ Get Started Free
Jordan
Patent: 0200119
Patent: مركبات مساعد مشترك من GIP وGLP-1 (GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 75
Patent: مركبات مساعد مشترك من GIP وGLP-1 (GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 242887
Estimated Expiration: ⤷ Get Started Free
Patent: 2023504
Estimated Expiration: ⤷ Get Started Free
Patent: 42887
Estimated Expiration: ⤷ Get Started Free
Luxembourg
Patent: 0296
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 3616
Patent: GIP AND GLP-1 CO-AGONIST COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 2753
Patent: COMPUESTOS CO-AGONISTAS DE GIP Y GLP-1. (GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 17008927
Patent: COMPUESTOS CO-AGONISTAS DE GIP Y GLP-1. (GIP AND GLP-1 CO-AGONIST COMPOUNDS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 21005835
Patent: COMPUESTOS CO-AGONISTAS DE GIP Y GLP-1. (GIP AND GLP-1 CO-AGONIST COMPOUNDS.)
Estimated Expiration: ⤷ Get Started Free
Moldova, Republic of
Patent: 42887
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 494
Patent: SPOJEVI KOJI SU SUAGONISTI GIP I GLP-1 (GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 315
Patent: COMPOSÉS CO-AGONISTES DE GIP ET DE GLP-1
Estimated Expiration: ⤷ Get Started Free
Patent: 422
Patent: COMPOSÉS CO-AGONISTES GIP ET GLP-1
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 1217
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 2000
Patent: Gip and glp-1 co-agonist compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 8274
Patent: Gip and glp-1 co-agonist compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 5618
Patent: Gip and glp-1 co-agonist compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 1043
Patent: Gip and glp-1 co-agonist compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 1547
Patent: Gip and glp-1 co-agonist compounds
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 23005
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 170954
Patent: COMPUESTOS CO-AGONISTAS DE GIP Y GLP-1
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 017501252
Patent: GIP AND GLP-1 CO-AGONIST COMPOUNDS.
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 42887
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 42887
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 146
Patent: JEDINJENJA KO-AGONISTI GIP I GLP-1 (GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201705603Y
Patent: GIP AND GLP-1 CO-AGONIST COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 42887
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1703930
Patent: GIP AND GLP-1 CO-AGONIST COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1957620
Estimated Expiration: ⤷ Get Started Free
Patent: 2330764
Estimated Expiration: ⤷ Get Started Free
Patent: 170092661
Patent: GIP 및 GLP-1 공효능제 화합물 (-1 GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 190026967
Patent: GIP 및 GLP-1 공효능제 화합물 (-1 GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 210145311
Patent: GIP 및 GLP-1 공효능제 화합물 (-1 GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 230023822
Patent: GIP 및 GLP-1 공효능제 화합물 (-1 GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 240135032
Patent: GIP 및 GLP-1 공효능제 화합물 (-1 GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 47928
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 82109
Estimated Expiration: ⤷ Get Started Free
Patent: 1636362
Patent: GIP and GLP-1 co-agonist compounds
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 17000198
Patent: GIP AND GLP-1 CO-AGONIST COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 8239
Patent: СПОЛУКА-КОАГОНІСТ GIP І GLP-1 (GIP AND GLP-1 CO-AGONIST COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ZEPBOUND around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Australia | 2013260666 | ⤷ Get Started Free | |
| South Africa | 201703930 | GIP AND GLP-1 CO-AGONIST COMPOUNDS | ⤷ Get Started Free |
| Portugal | 3242887 | ⤷ Get Started Free | |
| South Korea | 20240060873 | ⤷ Get Started Free | |
| Dominican Republic | P2017000153 | COMPUESTOS CO-AGONISTAS DE GIP Y GLP-1 | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ZEPBOUND
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3242887 | C202330010 | Spain | ⤷ Get Started Free | PRODUCT NAME: TIRZEPATIDA Y SALES FARMACEUTICAMENTE ACEPTABLES DE LA MISMA; NATIONAL AUTHORISATION NUMBER: EU/1/22/1685; DATE OF AUTHORISATION: 20220915; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/22/1685; DATE OF FIRST AUTHORISATION IN EEA: 20220915 |
| 3242887 | 2390005-3 | Sweden | ⤷ Get Started Free | PRODUCT NAME: TIRZEPATIDE AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REG. NO/DATE: EU/1/22/1685 20220919 |
| 3242887 | 2023C/506 | Belgium | ⤷ Get Started Free | PRODUCT NAME: TIRZEPATIDE; AUTHORISATION NUMBER AND DATE: EU/1/22/1685 20220919 |
| 3242887 | CR 2023 00005 | Denmark | ⤷ Get Started Free | PRODUCT NAME: TIRZEPATID OG FARMACEUTISK ACCEPTABLE SALTE DERAF; REG. NO/DATE: EU/1/22/1685 20220919 |
| 3242887 | CA 2023 00005 | Denmark | ⤷ Get Started Free | PRODUCT NAME: TIRZEPATID OG FARMACEUTISK ACCEPTABLE SALTE DERAF; REG. NO/DATE: EU/1/22/1685 20220919 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for ZEPBOUND
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
