Introduction: The Quid Pro Quo Recalibrated
In biopharmaceutical innovation a patent is more than a legal document; it is the foundational asset upon which entire companies are built, billions in capital are invested, and life-saving therapies are brought to market. For decades, the industry has operated under a specific understanding of the “patent bargain”—the quid pro quo where an inventor, in exchange for disclosing their invention to the public, receives a limited monopoly . But on May 18, 2023, the U.S. Supreme Court, in its unanimous decision in Amgen Inc. v. Sanofi, fired a shot across the bow of the entire sector, powerfully recalibrating the terms of that bargain .
The ruling, which invalidated Amgen’s broad patents for its cholesterol-lowering antibody, did not create new law. Instead, it reached back through a century of precedent to resurrect and reaffirm a simple, yet profound, principle: “the more one claims, the more one must enable” . This decision has fundamentally altered the strategic landscape for biologic patents, arming both innovator companies and biosimilar challengers with a new, sharper understanding of what it takes to claim, defend, and defeat a patent on a valuable biologic invention.
The commercial stakes could not be higher. The global biologics market is a behemoth, projected to soar past $612 billion by 2030, driven by groundbreaking therapies for cancer, autoimmune disorders, and rare diseases . Yet, this period of unprecedented growth coincides with a looming patent cliff of tectonic magnitude, with an estimated $200 billion to $300 billion in annual branded drug sales facing generic or biosimilar competition by the end of the decade . In this environment, a robust, defensible patent portfolio is not just an advantage; it is a matter of survival.
However, while the Amgen decision sent immediate shockwaves through the industry, it’s crucial to understand that this was not a sudden, unforeseen cataclysm. Rather, it was the powerful, definitive culmination of a tectonic shift in judicial philosophy that the U.S. Court of Appeals for the Federal Circuit had been signaling for over a decade. This trend, meticulously documented by legal scholars in a seminal 2021 paper titled “The Death of the Genus Claim,” revealed a consistent pattern of the Federal Circuit invalidating the very type of broad, functionally-defined “genus” claims that were once the bedrock of biopharma patent strategy . Amgen did not start this fire; it poured gasoline on it and cemented the new reality at the highest judicial level.
This report is designed to be your strategic playbook for navigating this new reality. We will move beyond a simple legal summary to provide a comprehensive analysis of the scientific, legal, and commercial implications of the Amgen ruling. For innovator companies, we will outline a forward-looking guide to drafting and prosecuting defensible biologic patents that can withstand the heightened scrutiny now required. For biosimilar developers, we will provide a clear framework for leveraging the Amgen precedent to challenge innovator patents, clear the path to market, and unlock a multi-billion dollar opportunity. The rules of the game have been clarified, and for those who understand them, the path to competitive advantage is now clearly illuminated.
Part I: The Foundations of the Enablement Doctrine
To fully grasp the strategic implications of the Amgen decision, we must first build a foundation of understanding upon two pillars: the complex science of antibody engineering and the core legal principles of patent disclosure. It was at the intersection of these two domains—the inherent unpredictability of biology and the law’s demand for predictability in disclosure—that Amgen’s patent empire crumbled.
A Tale of Two Proteins: The Science Behind the PCSK9 Showdown
At its heart, the dispute between Amgen and Sanofi was a battle over a biological mechanism that controls cholesterol in the human body. Understanding this science is not merely academic; it is the key to unlocking why the Supreme Court ruled as it did.
The Biology of ‘Bad’ Cholesterol
For millions of people, high levels of low-density lipoprotein (LDL) cholesterol—often called “bad” cholesterol—are a major risk factor for cardiovascular disease, heart attacks, and strokes . Our bodies have a natural system for managing this. Liver cells are dotted with LDL receptors, which act like molecular nets, capturing LDL cholesterol from the bloodstream and pulling it into the cell for disposal .
The efficiency of this system is regulated by a protein called proprotein convertase subtilisin/kexin type 9, or PCSK9. When PCSK9 binds to an LDL receptor, it effectively tags that receptor for destruction, preventing it from returning to the cell surface to capture more cholesterol . More PCSK9 activity means fewer LDL receptors, which in turn means higher levels of dangerous LDL cholesterol in the blood. This made PCSK9 an incredibly attractive therapeutic target: if you could block PCSK9, you could protect the LDL receptors and allow them to do their job more effectively.
The Ingenuity of Antibody Therapeutics
Both Amgen and Sanofi developed groundbreaking drugs to do just that. Their therapies, Repatha (evolocumab) and Praluent (alirocumab) respectively, are not traditional small-molecule pills but sophisticated biologics known as monoclonal antibodies . Antibodies are the body’s natural defenders, Y-shaped proteins designed to recognize and bind to specific targets (antigens) with exquisite precision. Scientists have harnessed this ability to create therapeutic monoclonal antibodies that can be engineered to bind to virtually any target of interest.
In this case, both companies engineered antibodies that could perform two critical functions: first, bind to a specific region of the PCSK9 protein, and second, by binding there, physically block PCSK9 from being able to attach to LDL receptors . The result was a dramatic and sustained reduction in LDL cholesterol levels, offering a powerful new treatment option for patients.
The Crux of the Matter: The Unpredictability of Antibody Engineering
Here we arrive at the scientific heart of the legal dispute. An antibody’s remarkable ability to bind to its target is dictated by its complex, three-dimensional structure, particularly in the regions at the tips of the “Y,” known as the complementarity-determining regions (CDRs). This 3D shape, in turn, is determined by the linear sequence of its building blocks, the amino acids .
The critical scientific fact that underpinned the entire Amgen case is this: the relationship between an antibody’s amino acid sequence and its final 3D structure and function is profoundly unpredictable . Changing just a single amino acid in a chain of hundreds can dramatically alter the antibody’s shape, potentially destroying its ability to bind to its target or, conversely, creating an entirely new function.
As the Supreme Court itself noted, citing expert testimony from the trial, scientists “cannot always accurately predict exactly how trading one amino acid for another will affect an antibody’s structure and function”. The task of translating a known amino acid sequence into a predicted 3D structure was described as a challenge that would “get a Nobel Prize for somebody at some point” but was “still not possible” at the time . This inherent unpredictability means that discovering a functional antibody is not a matter of rational design from first principles, but a process of generating vast numbers of candidates and then painstakingly screening them to find the rare few that work. It is this scientific reality that set Amgen on a collision course with a centuries-old legal doctrine.
The Court’s intense focus on the “unpredictability” of the art is not a static finding but a dynamic one. It implicitly creates a sliding scale for enablement that is directly tethered to the pace of technological progress. The ruling suggests that if and when technologies like artificial intelligence and machine learning can reliably and accurately predict an antibody’s 3D structure and binding function from its amino acid sequence, the entire premise of unpredictability could be revisited . In such a future, an inventor might be able to enable a broad genus of antibodies with far fewer examples, because the “roadmap” to creating them would no longer be one of trial and error, but of predictable, computational design. This means that today’s patent strategists must not only master the current legal standard but also keep a vigilant eye on the technological horizon. The very definition of what is “predictable” in your field could change, and with it, the breadth of the patent protection you can secure.
Decoding 35 U.S.C. § 112: The Enablement and Written Description Mandates
The patent bargain is codified in Section 112 of the U.S. Patent Act, which sets out the disclosure requirements an inventor must meet to receive a patent. Specifically, 35 U.S.C. § 112(a) contains three separate and distinct requirements that are often litigated: a written description of the invention, a description of how to make and use the invention (enablement), and the best mode for carrying out the invention . The Amgen case turned on the second of these: enablement.
Enablement vs. Written Description: A Critical Distinction
It is essential for any IP strategist to understand the difference between the enablement and written description requirements, as they provide two distinct avenues for challenging a patent’s validity.
- Enablement is forward-looking and instructive. It requires the patent specification to teach a Person of Ordinary Skill in the Art (a “POSA”) how to make and use the full scope of the claimed invention . The core question is: Does this patent provide a workable recipe?
- Written Description is backward-looking and evidentiary. It requires the specification to demonstrate that the inventor was in possession of the claimed invention as of the patent’s filing date . The core question is: Does this patent show that the inventor actually invented what they are now claiming?
A specification can, for example, describe a method of making a class of compounds (thus enabling them) without describing any specific compound with sufficient particularity to prove the inventor had “possession” of it. Conversely, an inventor could describe a specific chemical compound but fail to provide a method for making it, thus failing the enablement requirement. In the Amgen litigation, the patents were ultimately invalidated on enablement grounds, but both requirements are potent weapons in a challenger’s arsenal.
The “Undue Experimentation” Standard
The law does not demand that a patent specification be so exhaustive that a layperson can practice the invention without any effort. A “reasonable” amount of experimentation is permissible . The legal test, refined over decades of case law, is whether a POSA would have to engage in “undue experimentation” to make and use the invention across the full scope of the claims . What constitutes “undue” is not defined by a stopwatch or a budget; it is a qualitative assessment based on the nature of the invention and the state of the art.
The Enduring Relevance of the Wands Factors
To bring structure to this qualitative assessment, the Federal Circuit in its 1988 case, In re Wands, established a set of eight factors to be weighed when determining if experimentation is “undue” . While the Supreme Court in Amgen conspicuously avoided mentioning Wands by name, its reasoning implicitly touched on many of the same concepts . More importantly, the U.S. Patent and Trademark Office (USPTO) issued formal guidance in January 2024 explicitly stating that the Wands factors remain the operative framework for patent examiners assessing enablement across all technologies . This makes a deep understanding of these factors non-negotiable for anyone prosecuting or litigating a biologic patent today.
For clarity and strategic application, these factors serve as the practical checklist for building an enablement case or defending against one.
Table 1: The In re Wands Factors for Determining Undue Experimentation
| Factor | Description and Strategic Consideration |
| (A) The Breadth of the Claims | How broad is the claimed genus? A claim covering millions of potential antibodies, like Amgen’s, requires a much stronger enabling disclosure than a claim to a single antibody sequence. This factor was central to the Amgen decision. |
| (B) The Nature of the Invention | Is the invention in a complex and unpredictable field, like antibody engineering, or a more predictable one, like mechanical engineering? The more unpredictable the art, the more disclosure is required. |
| (C) The State of the Prior Art | What was already known to the public when the patent was filed? A specification can rely on established, well-known techniques without having to explain them from scratch. |
| (D) The Level of One of Ordinary Skill | Who is the POSA? A highly skilled molecular biologist is expected to be able to fill in more gaps than a novice, which can lessen the disclosure burden. |
| (E) The Level of Predictability in the Art | This is a critical factor and overlaps with the “Nature of the Invention.” Can a POSA reliably predict the outcome of modifying the invention? For antibodies, the Supreme Court found the art highly unpredictable. |
| (F) The Amount of Direction Provided | How much guidance does the patent provide? A detailed “roadmap” with specific protocols and parameters is far more enabling than a mere suggestion to engage in trial-and-error screening. |
| (G) The Existence of Working Examples | Does the patent describe actual experiments that were performed and worked? The presence, number, and diversity of working examples are powerful evidence of enablement. Amgen’s 26 examples were deemed insufficient for its broad claims. |
| (H) The Quantity of Experimentation Needed | This is the ultimate question. Considering all the other factors, how much work would a POSA have to do to practice the full scope of the invention? Is it a reasonable, iterative process or a “painstaking” and open-ended research project? |
Understanding these factors is the first step in building a post-Amgen patent strategy. They provide the vocabulary and the analytical framework that the USPTO and the courts will use to judge whether your patent has upheld its end of the bargain.
Part II: Dissecting the Decision: “The More You Claim, The More You Must Enable”
The Supreme Court’s opinion in Amgen v. Sanofi is a masterclass in judicial reasoning, notable not just for what it says, but for how it says it. By sidestepping modern patent jurisprudence in favor of foundational, century-old cases, the Court delivered a ruling that is both a powerful affirmation of the Federal Circuit’s recent trajectory and a timeless statement on the core principles of patent law.
Anatomy of a Landmark Case: From Infringement Suit to Supreme Court
The legal battle began in 2014, when Amgen sued Sanofi for infringing two of its patents, U.S. Patent Nos. 8,829,165 and 8,859,741, with its competing PCSK9 inhibitor, Praluent . The case centered on a specific type of patent claim known as a “genus” claim.
The Patents-in-Suit and the Functional Genus Claim
Unlike a “species” claim, which covers a single, specific chemical structure (like the exact amino acid sequence of one antibody), a “genus” claim covers an entire family or class of related structures. Amgen’s patents went a step further, employing functional genus claims. The claims did not define the antibodies by their structure, but by what they did:
- Bind to a specific set of amino acid residues on the PCSK9 protein.
- Block PCSK9 from binding to the LDL receptor .
A representative claim (claim 19 of the ‘165 patent) reads: “The isolated monoclonal antibody of claim 1 wherein the isolated monoclonal antibody binds to at least two of the following residues S153, I154, P155, R194, D238, A239, I369, S372, D374, C375, T377, C378, F379, V380, or S381 of PCSK9 listed in SEQ ID NO:3” . This claim language is purely functional. It laid claim to potentially millions of different antibody molecules—regardless of their sequence—so long as they performed the specified binding and blocking functions . In its patent specification, Amgen disclosed the specific amino acid sequences for 26 antibodies that met this functional definition . The legal question was whether disclosing 26 species was enough to lay claim to the entire, vast genus.
The Litigation Journey: A Rollercoaster Ride to Invalidation
The path to the Supreme Court was long and convoluted, highlighting the high stakes and legal complexities.
- An initial jury trial in 2016 found Amgen’s patents were not invalid, and the court entered a permanent injunction against Sanofi’s Praluent.
- The Federal Circuit vacated that injunction and remanded for a new trial, finding that the district court had improperly excluded certain evidence related to enablement .
- A second jury in 2019 again found in Amgen’s favor, concluding Sanofi had failed to prove the patents were invalid .
- The turning point came when the district court judge took the extraordinary step of granting Sanofi’s motion for judgment as a matter of law (JMOL), effectively overturning the jury’s verdict. The judge ruled that, based on the undisputed facts, no reasonable jury could find the patents were enabled .
- The Federal Circuit affirmed the district court’s JMOL, setting the stage for the final appeal to the Supreme Court .
This procedural history is itself a crucial lesson: in patent law, enablement is ultimately a question of law based on underlying facts. Even two favorable jury verdicts could not save patents that a judge determined were legally deficient on their face.
The Core Arguments at the Supreme Court
When the case reached the high court, the arguments were sharply defined, representing two fundamentally different views of the patent bargain.
- Amgen’s Position: Amgen argued that its specification was fully enabling. It provided a “roadmap” for scientists to follow: generate a pool of candidate antibodies using standard techniques (like immunizing mice) and then screen them for the desired binding and blocking functions . It also proposed a “conservative substitution” method, where scientists could start with the 26 known antibodies and swap out individual amino acids for structurally similar ones to create new variants . Amgen contended that the Federal Circuit had created a new, unworkable standard that required an inventor to make and test every single embodiment of a claimed genus, which would stifle breakthrough inventions .
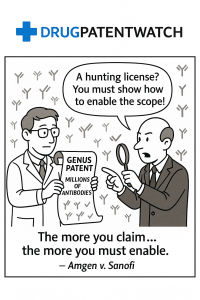
- Sanofi’s Position: Sanofi, supported by the U.S. Solicitor General, countered that Amgen’s “roadmap” was nothing more than an invitation for “trial and error” or, more pointedly, a “hunting license” to conduct a research project that Amgen itself had not completed . Given the profound unpredictability of antibody science, there was no way to know which of the millions of potential candidates would work without exhaustive screening. They argued that by claiming a monopoly over a vast, unexplored territory based on planting a flag on just 26 small plots of land, Amgen had failed to provide its quid for the enormous quo it demanded .
Inside the Unanimous Opinion: A Masterclass in Precedent
In a concise and powerfully written opinion by Justice Neil Gorsuch, the unanimous Court sided decisively with Sanofi, affirming the Federal Circuit’s judgment . The opinion’s brilliance lies in its simplicity and its strategic reliance on foundational precedent.
The Central Holding: A Simple Statutory Command
The Court distilled the complex legal arguments down to a single, clear principle derived directly from the statute and historical case law: “If a patent claims an entire class of processes, machines, manufactures, or compositions of matter, the patent’s specification must enable a person skilled in the art to make and use the entire class. In other words, the specification must enable the full scope of the invention as defined by its claims” . This led to the now-famous maxim: “The more one claims, the more one must enable” .
Weaponizing History: Morse, Incandescent Lamp, and Holland Furniture
Rather than engaging with the Federal Circuit’s modern, factor-based Wands test, Justice Gorsuch reached back into the Court’s own history, resurrecting three seminal cases that dealt with overbroad, functional claims in different technological eras . This was a deliberate choice, designed to frame the enablement principle as timeless and universal.
- O’Reilly v. Morse (1854): The Court recalled how it invalidated Samuel Morse’s eighth claim in his telegraph patent, which attempted to claim not just his specific device but the very principle of using “electro-magnetism, however developed for marking or printing intelligible characters… at any distances” . The Court found Morse had not enabled every possible way of achieving this result.
- The Incandescent Lamp Patent (1895): The Court cited the case where inventors who had discovered that carbonized paper could be used as a filament for a light bulb tried to claim all “carbonized fibrous or textile material.” Their claim was invalidated because it was Thomas Edison who, through “painstaking experimentation,” discovered that bamboo was a far superior material, a discovery not taught by the original patent .
- Holland Furniture Co. v. Perkins Glue Co. (1928): The Court pointed to its invalidation of a patent for a starch-based glue that claimed not just the specific formulation but all starch glues having “substantially the same properties as animal glue.” This was too broad because it would require others to engage in “elaborate experimentation” to find out which other starches worked .
By drawing a direct line from Morse’s telegraph to Edison’s light bulb to Amgen’s antibodies, the Court accomplished two critical goals. First, it sidestepped any debate about the nuances of the Wands factors, elevating the core principle above the specific test. Second, it powerfully refuted any argument that biologics or other complex modern technologies deserve a special, more lenient standard—a concept known as “tech-exceptionalism” . The message was clear: the fundamental rules of the patent bargain apply equally to 19th-century electrical engineering and 21st-century biotechnology. This judicial maneuver gives implicit approval to the Federal Circuit’s stricter application of enablement principles while grounding that approval in the Supreme Court’s own unassailable, historical authority. For litigators, this means that arguments against broad claims should now be framed not only in the language of Wands but also by drawing direct analogies to this powerful trio of historical precedents.
The “Hunting License” and the “General Quality” Exception
Applying this historical framework, the Court found that Amgen’s claims were directly analogous to these past overreaches. Amgen’s “roadmap” and “conservative substitution” methods were dismissed as “little more than two research assignments” . They did not teach a POSA how to reliably create the claimed antibodies but instead invited them to “engage in ‘painstaking experimentation’ to see what works,” which the Court concluded “is not enablement. More nearly, it is ‘a hunting license'” .
However, the Court did not shut the door on genus claims entirely. Citing Incandescent Lamp, it noted that a specification can enable a broad class with just a few examples, but only if it also discloses “some general quality running through the class that gives it a peculiar fitness for the particular purpose” . This “general quality” would be a unifying principle—perhaps a common structural motif or a predictable structure-activity relationship—that would allow a POSA to reliably predict which other members of the class would also work. Amgen, the Court found, had failed to identify any such quality for its claimed antibodies.
Part III: The Ripple Effect: How Courts Are Applying the Amgen Standard
A Supreme Court decision is not an endpoint; it is the start of a new chapter in legal interpretation. In the months following the Amgen ruling, the lower courts and the USPTO have begun to apply its principles, providing a clearer picture of the new enablement landscape. The emerging trend is one of strict application, confirming that Amgen has provided a powerful and straightforward template for invalidating overly broad claims, not just for antibodies, but across a range of technologies.
The Federal Circuit Follows Through: Baxalta and Medytox
The Federal Circuit, whose own jurisprudence was affirmed by the Supreme Court, has moved swiftly to apply the Amgen standard in subsequent cases. Two decisions in particular, Baxalta and Medytox, are instructive for their directness and for what they reveal about the ruling’s broader impact.
Baxalta Inc. v. Genentech, Inc.: The “Materially Indistinguishable” Antibody Case
Decided just four months after Amgen, the Baxalta case presented a factual scenario that was a near-perfect echo of the PCSK9 dispute.
- The Technology: Baxalta’s patent (U.S. Patent No. 7,033,590) was for a hemophilia treatment and claimed a genus of antibodies defined by their dual functions: binding to the blood clotting enzymes Factor IX or Factor IXa, and increasing the procoagulant activity of Factor IXa .
- The Disclosure: The patent specification disclosed the amino acid sequences of eleven specific antibodies that performed these functions and, like Amgen’s patent, provided a “roadmap” for creating more using standard hybridoma and screening techniques .
- The Ruling: The Federal Circuit’s analysis was swift and decisive. It declared the facts of the case to be “materially indistinguishable from those in Amgen”. The court noted that Baxalta’s claims covered a potentially vast number of antibodies, yet the specification provided only a handful of examples and a trial-and-error method for finding more. Crucially, the court pointed out that the patent failed to disclose any “quality common to every functional embodiment” that would allow a POSA to predict which other antibodies would work without having to screen them all. The claims were therefore held invalid for lack of enablement.
The Baxalta decision demonstrates that the Federal Circuit is not treating Amgen as a nuanced standard requiring a deep, case-by-case re-weighing of the Wands factors. Instead, it is applying it as a bright-line rule. If a patent claims a genus of antibodies by function, provides a small number of examples, and relies on a screening “roadmap” in an unpredictable field, it is highly likely to be found invalid. This creates a powerful analytical shortcut for challengers: framing an opponent’s patent as “Amgen-like” is now the most direct path to summary judgment of invalidity. For innovators, it is a stark confirmation that the old way of claiming is no longer viable.
Medytox, Inc. v. Galderma S.A.: Extending Amgen Beyond Antibody Structure
The Medytox case is arguably even more significant than Baxalta because it shows the Amgen principle extending beyond claims to antibody structures and into the realm of functional outcomes and numerical ranges.
- The Technology: Medytox’s patent (U.S. Patent No. 10,143,728) was directed to a botulinum toxin (Botox-like) composition. In a post-grant review proceeding, Medytox sought to amend its claims to include a functional limitation: a method of treatment that results in a “responder rate at 16 weeks after the first treatment of 50% or greater” .
- The Disclosure: The patent specification provided only three working examples with responder rates above 50%: specifically, 52%, 61%, and 62% . It offered no guidance or scientific principle explaining how a POSA could achieve a responder rate at the higher end of the claimed scope, such as 80%, 90%, or 100%.
- The Ruling: The Federal Circuit construed the “50% or greater” limitation as a range from 50% to 100% . It then directly invoked the central tenet from Amgen: “[t]he more one claims, the more one must enable” . The court found that because Medytox’s specification did not enable the full scope of the claimed range—providing no path to achieve the higher responder rates—the claims were invalid for lack of enablement .
This decision is a critical data point for all biopharma patent strategists. It confirms that the Amgen logic is not confined to genus claims for molecules. It applies with equal force to any claim that defines an invention by a functional outcome or a numerical range. If you claim a therapeutic effect up to a certain level, you must provide a disclosure that enables a POSA to achieve that effect across the entire claimed range. Simply providing a few data points at the lower end will not suffice.
Beyond Biologics: In re Starrett and the Universal Standard
To erase any doubt that the Amgen standard was limited to the life sciences, the Federal Circuit and the USPTO have pointed to In re Starrett as evidence of its universal applicability .
- The Technology: The patent application at issue was far from biotechnology; it claimed a “non-transitory computer readable medium for maintaining augmented telepathic data” .
- The Disclosure and Ruling: The claims contained forty-seven “or” clauses, which the Patent Trial and Appeal Board (PTAB) calculated could cover over 140 trillion different embodiments . The Board, applying the Wands factors, found the claims were not enabled. The Federal Circuit affirmed this rejection.
- The Significance: The USPTO’s own post-Amgen guidance explicitly cites Starrett to make the point that the principles from Amgen and the Wands framework are to be applied “regardless of the technology” . This confirms the Supreme Court’s strategic effort to frame its decision in timeless, technology-neutral terms was successful. The heightened scrutiny on enablement is the new normal for all fields of invention.
The View from the PTAB: A New Scrutiny
The PTAB, the administrative body within the USPTO that hears challenges to issued patents, has also quickly integrated Amgen into its jurisprudence. A review of PTAB decisions since May 2023 shows a clear trend: Amgen is being used as a powerful tool to invalidate claims that are seen as overreaching their disclosure .
Decisions have affirmed enablement rejections for a wide array of technologies, including:
- Chimeric T-cell antigen receptors (TCRs) .
- Methods of genetically modifying fruit flies .
- Methods of treating diseases using broad classes of “small-sized molecules” .
- Methods of stimulating an immune response using mRNA encoding a coronavirus spike protein .
In several of these cases, particularly those involving broad functional claims in biology, the PTAB has adopted the Federal Circuit’s analytical shortcut. Rather than conducting a full, eight-factor Wands analysis from scratch, the Board simply compares the applicant’s specification to Amgen’s, finds it to be similarly or even more deficient in its guidance, and concludes the claims are not enabled by direct analogy . This further solidifies Amgen‘s status as a go-to invalidity template, streamlining the path for both patent examiners and third-party challengers to reject or invalidate broad functional claims.
Part IV: The Strategic Playbook for Innovator Companies
In the wake of Amgen, the strategic imperative for innovator companies has shifted dramatically. The era of securing a dominant market position with a single, broad functional genus claim is over. The new paradigm demands a more sophisticated, data-driven, and multi-layered approach to patent prosecution. The goal is no longer to simply claim a kingdom, but to build a defensible fortress, claim by claim, brick by brick. This requires a fundamental change in mindset, starting long before a patent application is ever drafted.
Drafting Bulletproof Biologic Patents in a Post-Amgen World
The value of a biologic patent is now directly proportional to the quality and depth of its specification. A specification can no longer be a mere outline of an idea; it must be, as Amgen’s counsel argued (but failed to deliver), a “rich handbook” for the POSA.
The Data-Rich Specification: Your First Line of Defense
The most critical takeaway from Amgen is that data is king. A patent application for a biologic must be supported by a robust and diverse experimental record. This includes:
- Extensive Working Examples: The number of actual, reduced-to-practice examples is paramount. While there is no magic number, the quantity and diversity of examples must be commensurate with the desired claim scope . If you want to claim a family of antibodies that bind to different parts of an epitope, you must provide examples that do so. If you want to claim a range of sequence variations, you must provide examples showing that those variations maintain function. Amgen’s 26 examples were deemed woefully insufficient to support a claim covering millions of potential antibodies.
- Detailed Protocols: The specification should include detailed, step-by-step protocols for making and testing the invention. Vague references to “standard techniques” are no longer enough. Provide specific parameters, reagent concentrations, and assay conditions to demonstrate that the process is controlled and repeatable.
- Structural and Functional Characterization: For antibodies, this means providing as much data as possible: full amino acid sequences (heavy and light chain), the sequences of all six CDRs, binding affinity data (e.g., KD), off-rates (koff), epitope mapping data identifying the specific residues contacted, and data from functional assays (e.g., blocking, neutralizing, or activating). Including 3D structural data from techniques like X-ray crystallography, as Amgen did for two of its antibodies, is also highly valuable .
Working vs. Prophetic Examples: A New Risk Calculus
Patent law allows for two types of examples: “working” examples based on experiments actually performed, and “prophetic” examples describing experiments that are reasonably expected to work but have not yet been conducted . Post-Amgen, the strategic value of these two types has diverged significantly.
- Working Examples are Gold: As discussed, these are the bedrock of an enabling disclosure. They provide concrete proof that the inventor has made and used at least part of the claimed invention.
- The Diminished Role of Prophetic Examples: Prophetic examples can still be useful for illustrating the potential breadth of an invention and are a common practice . However, they must be drafted with extreme care—always in the present or future tense to avoid any implication that the work has already been done, which can lead to charges of inequitable conduct. In a highly unpredictable art like antibody engineering, the ability of prophetic examples to support the full scope of a broad claim is now severely limited. Relying on them to bridge the gap between a few working examples and a vast functional genus is a high-risk strategy that is likely to fail under Amgen‘s scrutiny.
Claim Layering and Diversification: The “Picture Frame” Strategy
The “all eggs in one basket” approach of relying on a single broad genus claim is now indefensibly risky. The modern strategy is one of defense-in-depth, building layers of claims with varying scope, much like a set of nested picture frames around the core invention. This ensures that even if the broadest claims are invalidated, the innovator is left with a core of narrower, defensible claims that can still provide meaningful market protection.
A robust antibody patent application should include a diverse portfolio of claims:
- The Innermost Frame (Species Claims): These are the narrowest and strongest claims. They should be directed to the specific antibodies that have been made and tested. This includes claims reciting the full amino acid sequence of the heavy and light chains, or at a minimum, the sequences of the six CDRs that are primarily responsible for antigen binding. These claims are the most likely to survive an enablement challenge.
- The Intermediate Frame (Limited Genus Claims): The next layer can broaden the scope slightly. These claims might recite a certain percentage of sequence identity to an exemplified antibody (e.g., “an antibody comprising a heavy chain variable region having at least 95% sequence identity to SEQ ID NO:1”) or allow for a limited number of “conservative” amino acid substitutions . Crucially, to support these claims, the specification should include data demonstrating that antibodies with this degree of variation actually retain the desired function. Without such data, these claims are vulnerable to the same “trial-and-error” arguments that doomed Amgen’s.
- The Outermost Frame (Broad Functional Claims): This is now the highest-risk, highest-reward layer. A broad functional genus claim should only be pursued if the inventor can satisfy the “general quality” exception articulated by the Supreme Court. This requires identifying and disclosing a common structural feature or a predictable structure-activity relationship that reliably confers the desired function across the entire claimed class. For example, if an inventor discovers that any antibody with a specific structural loop in its CDR3 and a particular charge at a key position will block a target, that might constitute an enabling “general quality.” Without such a unifying principle, a broad functional claim is likely a “hunting license” awaiting invalidation.
The Viability of Alternative Claiming Strategies
Given the new landscape, patent prosecutors must be creative and explore alternative claiming formats that may offer a path to broader protection.
Structure-Function Claims: The New Gold Standard
The most prudent path forward is the hybrid structure-function claim. Rather than claiming only by function, these claims tether the function to specific structural elements. For an antibody, this would mean claiming:
- An antibody that binds to epitope X (function) and comprises heavy chain CDRs 1, 2, and 3 as set forth in SEQ ID NOs: Y, Z, and A (structure) .
This format provides significantly more support than a pure functional claim. It puts the public on notice of the key structural features responsible for the invention’s activity, while still providing some breadth to capture variants outside of the non-essential “framework” regions of the antibody.
The Means-Plus-Function Gambit (35 U.S.C. § 112(f))
A more novel and largely untested strategy for antibodies is the use of “means-plus-function” claiming under 35 U.S.C. § 112(f) .
- How It Works: This provision allows an inventor to claim an element of their invention as a “means for” performing a function, without reciting the specific structure that performs the function in the claim itself . For example, a claim could recite a “means for binding human C5 protein” .
- The Scope: The scope of such a claim is statutorily defined. It is construed to cover the corresponding structure described in the specification and its equivalents . This is the key potential advantage: it may capture not only the exact antibody disclosed but also other, structurally different antibodies that perform the same function in substantially the same way to achieve the same result.
- The Ex parte Chamberlain Opening: This strategy recently gained a foothold at the USPTO. In a case involving an antibody claim drafted in this format, an Appeals Review Panel clarified that while the specification must disclose the corresponding structure (e.g., the specific antibody), it does not need to describe all of the potential equivalents of that structure . This suggests that means-plus-function may be a viable route to obtaining broader claim scope than is possible with a traditional structural claim.
- The Caveats: This strategy is still nascent and carries risks. The specification must still provide robust structural support for the “means”. Furthermore, the scope of “equivalents” in the highly unpredictable field of antibody engineering is an open question that will undoubtedly be heavily litigated. While it is a creative tool that prosecutors should now consider including in their claim sets, it is far from a proven magic bullet to bypass the core challenges of Amgen.
Part V: The Challenger’s Advantage: A Guide for Biosimilar Developers
While the Amgen decision presents a formidable challenge for innovator companies, it represents a golden opportunity for biosimilar developers. The Supreme Court has effectively handed challengers a powerful, streamlined weapon for invalidating the broad, functionally-defined patents that have historically formed the thickest part of the “patent thickets” protecting blockbuster biologics. For the savvy biosimilar company, the strategic landscape has never been more favorable.
Identifying the Weakest Link: A Guide to Targeting Vulnerable Patents
The first step in any successful biosimilar strategy is to identify and prioritize the innovator patents that are most vulnerable to a validity challenge. Post-Amgen, this process has become much more systematic. Biosimilar developers should actively screen the patent portfolios of their target reference products using what can be called the “Amgen Litmus Test.”
Patents that are prime targets for an enablement challenge will exhibit most or all of the following characteristics:
- Broad, Functionally-Defined Genus Claims: The claims define the invention by what it does (e.g., “an antibody that blocks binding of protein X to receptor Y”) rather than what it is (e.g., “an antibody comprising the six CDRs of SEQ ID NOs: 1-6”).
- Invention in an Unpredictable Art: The patent is in a field acknowledged to be unpredictable, with antibody patents being the quintessential example. This also applies to other complex biologics like CAR-T therapies or novel gene-editing systems.
- High Claim Scope-to-Example Ratio: The patent discloses a relatively small number of working examples (like Amgen’s 26) while the functional claims can be credibly argued to cover thousands or even millions of potential embodiments.
- A “Roadmap” Disclosure: The specification’s method for creating additional embodiments relies on a generic, trial-and-error screening process (a “roadmap”) rather than a predictable, structure-based design principle (a “general quality”).
- Older Priority Date: Patents filed and granted in the pre-Amgen era, when the USPTO and courts were more lenient toward broad genus claims, are particularly vulnerable. They were written under an old set of assumptions that have now been definitively invalidated.
Operationalizing Intelligence with DrugPatentWatch
This screening process can be powerfully augmented by leveraging sophisticated patent intelligence platforms. A tool like DrugPatentWatch is no longer just a defensive instrument for freedom-to-operate (FTO) analysis; it is an offensive weapon for identifying strategic targets . A biosimilar company can use such a platform to:
- Systematically Monitor Competitor Portfolios: Track all patents associated with a target biologic, including newly issued patents and pending applications.
- Filter for “Amgen-like” Claims: Search patent databases for keywords indicative of broad functional claims (e.g., “binds,” “blocks,” “inhibits,” “modulates”) associated with key biologic targets.
- Analyze Patent Specifications: Once a potentially vulnerable patent is identified, the platform can provide access to the full document, allowing legal and scientific teams to analyze the number of working examples, the disclosure of any “roadmap,” and the breadth of the claims.
- Track Precedent-Setting Litigation: Monitor ongoing patent litigation across the industry to see how other companies are successfully deploying Amgen-based arguments, providing valuable insights for your own legal strategy.
By systematically applying the Amgen litmus test and operationalizing it with a powerful intelligence tool, a biosimilar developer can shift from a reactive posture to a proactive one, strategically targeting the weakest patents in an innovator’s fortress to clear a faster, less expensive path to market.
Weaponizing Enablement in BPCIA Litigation and PTAB Challenges
Once a vulnerable patent is identified, the Amgen precedent provides a clear roadmap for challenging it. Enablement is no longer a secondary, fact-intensive defense; it is a primary, often dispositive, legal argument that can be deployed early and effectively.
The New Center of Gravity in District Court Litigation
For biosimilar companies facing an infringement suit, an Amgen-based enablement defense should be the centerpiece of their invalidity case . The key strategic advantage is that it can often be resolved at the summary judgment stage. Because the core issue is a matter of law—whether the specification, on its face, enables the full scope of the claims—a challenger can argue that no reasonable jury could find the patent valid, potentially avoiding a long and expensive trial on more complex factual issues like infringement.
Altering the Calculus of the “Patent Dance”
The Biologics Price Competition and Innovation Act (BPCIA) created a complex, pre-litigation information exchange process known as the “patent dance,” where the biosimilar applicant and the reference product sponsor exchange lists of patents that may be infringed . While the Supreme Court has clarified that this dance is largely optional, the Amgen decision fundamentally alters the strategic calculus for those who participate. A biosimilar company can now enter these negotiations with a powerful bargaining chip. By clearly signaling its intent to mount a vigorous Amgen-based invalidity challenge against the innovator’s broadest functional claims, the biosimilar developer can increase its leverage, potentially leading to a more favorable settlement or convincing the innovator to drop its weakest and most valuable patents from the ensuing litigation.
The PTAB as a Killing Ground for Weak Patents
Perhaps the most effective venue for a biosimilar challenger is the Patent Trial and Appeal Board (PTAB). A Post-Grant Review (PGR), which can be filed within nine months of a patent’s issuance, allows for challenges on any ground of invalidity, including lack of enablement under § 112. This makes it an ideal forum for an Amgen-based attack.
The statistics on PTAB challenges to biologic patents paint a stark and compelling picture for biosimilar developers.
Table 2: PTAB Invalidation Outcomes for Biologic Patents (Cumulative, 2012-2021)
| Metric | Statistic | Strategic Implication |
| All-Time Institution Rate | 55% | Lower than the average for all technologies (64%). Getting a challenge started is the biggest hurdle. |
| % of FWDs with All Claims Invalidated | 70% | Extremely high. Once a petition is instituted, the odds of success for the challenger are overwhelming. |
| % of FWDs with All Claims Patentable | 21% | Very low. The chances of the patent owner emerging from a trial unscathed are slim. |
| % of Instituted Claims Found Unpatentable | 59% | A majority of individual claims that go to trial are ultimately cancelled. |
Source: Synthesized from data presented in studies analyzing USPTO PTAB statistics . FWD = Final Written Decision.
This data tells a clear story. While the PTAB is selective about which petitions it institutes for biologic patents, once that threshold is crossed, the forum is exceptionally favorable to the challenger. The combination of this statistical advantage with the powerful legal precedent of Amgen creates an almost irresistible business case for biosimilar companies to be aggressive. A well-crafted PGR petition that clearly lays out how a target patent fails the Amgen standard has a very high probability of success, making the PTAB a cost-effective and efficient tool for clearing key blocking patents.
Part VI: The Road Ahead
The Supreme Court’s decision in Amgen v. Sanofi was not the creation of a new legal world, but a definitive clarification of the existing one. It has swept away the ambiguity that allowed broad, functional claims to proliferate in the unpredictable biologic arts and replaced it with a bright-line standard rooted in the fundamental patent bargain. The era of claiming an entire functional kingdom based on the discovery of a single key is over. For innovators and challengers alike, this new clarity presents both significant challenges and profound opportunities.
Conclusion: Navigating the Post-Amgen Paradigm
For innovator companies, the path forward demands a deeper integration of patent strategy and scientific discovery. The value of a biologic invention will now be captured not by a single, sweeping claim, but through a meticulously constructed portfolio of layered, structurally-grounded claims supported by a wealth of empirical data. The specification is no longer a formality; it is the fortress. Success will belong to those who invest in generating the robust data packages needed to justify their claims and who embrace creative, defense-in-depth claiming strategies. The R&D process itself must be reoriented to not only discover a functional molecule but also to elucidate the underlying principles—the “general qualities”—that make it work.
For biosimilar developers, the landscape is ripe with opportunity. The Amgen decision has provided a powerful and efficient tool for dismantling the patent thickets that have long delayed the entry of lower-cost biologics. The strategic imperative is to shift from a defensive, freedom-to-operate mindset to an offensive one. By systematically identifying and challenging vulnerable patents—both in district court and at the PTAB—biosimilar companies can accelerate their path to market, increase competition, and deliver substantial cost savings to the healthcare system. The challenger’s advantage has never been greater.
Ultimately, the Amgen ruling forces the entire biopharmaceutical industry to return to first principles. It reaffirms that a patent is not a reward for identifying a problem, but for delivering a concrete and teachable solution. In this recalibrated paradigm, the companies that thrive will be those that not only make groundbreaking discoveries but also take on the rigorous work of truly understanding and enabling them for the benefit of all.
Key Takeaways
- The Core Principle is “Scope Commensurate with Enablement”: The Supreme Court’s central holding, “the more one claims, the more one must enable,” is now the definitive standard. All patent strategies must be built around this principle.
- Functional Genus Claims are High-Risk: Relying on claims that define a biologic (especially an antibody) solely by its function is now an extremely risky strategy. Such claims are prime targets for invalidation.
- The Specification is Paramount: The strength of a patent now lies almost entirely in the depth and quality of its specification. A data-rich disclosure with numerous, diverse working examples is the best defense against an enablement challenge.
- Adopt a Layered “Picture Frame” Claiming Strategy: The most robust patent portfolios will include a mix of claims: narrow species claims (by sequence), intermediate claims (by % identity), and broader claims that are explicitly tied to disclosed structural features.
- “Unpredictability” is the Key Factual Battleground: For both innovators and challengers, the central factual question in an enablement dispute will be the predictability of the art. As technology (like AI-driven protein design) evolves, so too will the application of the Amgen standard.
- Biosimilar Challengers Have a Powerful New Weapon: Amgen provides a clear, streamlined path to challenge and invalidate broad innovator patents, particularly at the summary judgment stage in district court or via a Post-Grant Review at the PTAB.
- The Wands Factors Remain the Operative Test: Despite the Supreme Court’s silence on Wands, the USPTO and the Federal Circuit have confirmed that the eight Wands factors remain the practical framework for assessing “undue experimentation.”
Frequently Asked Questions (FAQ)
1. Does Amgen v. Sanofi mean I can no longer get a patent on a groundbreaking antibody discovery?
Not at all. The ruling does not prevent the patenting of antibodies. In fact, the Supreme Court explicitly stated that Amgen’s claims to the 26 specific antibodies it fully described by amino acid sequence were not in doubt. What the decision curtails is the ability to leverage the discovery of a few antibodies into a broad monopoly over every potential antibody that performs the same function. You can absolutely patent what you have actually invented and enabled. The key is to ensure your claim scope is commensurate with your disclosure.
2. How many working examples are now “enough” to support a genus claim?
There is no “magic number.” The analysis is qualitative, not quantitative, and depends heavily on the Wands factors, especially the breadth of the claim and the predictability of the art. For a very narrow genus in a predictable field, a single example might suffice. For a broad genus of antibodies covering millions of potential members in a highly unpredictable field, even Amgen’s 26 examples were not enough. The strategic answer is to provide as many diverse examples as is feasible to illustrate the breadth of your invention and, if possible, to identify a common structural or chemical principle that links them.
3. Will advances in AI and machine learning eventually reverse the impact of Amgen on antibody patents?
This is a distinct possibility and a critical area to watch. The Supreme Court’s reasoning was heavily dependent on the “unpredictable” nature of antibody engineering . If AI and computational models evolve to the point where they can reliably predict an antibody’s 3D structure and function from its sequence, the art would become far more “predictable” . In such a scenario, an inventor might be able to enable a broad functional genus with far fewer examples, because the “roadmap” would no longer be trial-and-error but a predictable, in silico design process. The Amgen standard would still apply, but meeting it would become easier.
4. I have a portfolio of older patents with broad functional claims. What are my options now?
You have several strategic options, each with its own risks and benefits.
- Hold and Defend: You can wait until the patent is challenged and then defend it, arguing that your specific technology is more predictable than antibody science or that your disclosure provides the requisite “general quality.” This is a high-risk strategy.
- File for Reissue: If the patent is still within the two-year window for broadening reissue, or at any time for a narrowing reissue, you could attempt to add narrower, structurally-defined claims that are better supported by the original specification.
- Strengthen with Continuations: If you have a pending continuing application in the same family, you should aggressively prosecute narrower, layered claims in that application to build a defensive moat around the core invention.
- Proactive Pruning: In some cases, it may be strategic to review your portfolio, identify the patents most vulnerable under Amgen, and consider allowing them to lapse to save on maintenance fees, focusing resources on more defensible assets.
5. As a biosimilar company, how do I balance the cost of a PTAB challenge against the potential reward, even with the Amgen precedent?
The cost-benefit analysis has shifted significantly in the biosimilar’s favor. While a PTAB challenge (especially a PGR) is a significant investment (often in the high six to low seven figures), it must be weighed against the cost of years of district court litigation and the massive revenue potential of entering a blockbuster biologic market years earlier. The statistics are compelling: once instituted, biologic patents are invalidated at the PTAB at a very high rate. Amgen provides a clear, legally powerful argument that increases the likelihood of both institution and ultimate success. Therefore, for a key blocking patent that fits the Amgen vulnerability profile, a PTAB challenge represents a high-probability, high-reward strategic investment that can yield a return many orders of magnitude greater than its cost.
References
- Amgen Inc. v. Sanofi – Food and Drug Law Institute (FDLI), accessed August 7, 2025, https://www.fdli.org/2024/05/amgen-inc-v-sanofi/
- Amgen Inc. v. Sanofi – SCOTUSblog, accessed August 7, 2025, https://www.scotusblog.com/case-files/cases/amgen-inc-v-sanofi-2/
- Amgen v. Sanofi: The U.S. Supreme Court Reviews Patent Enablement – PubMed, accessed August 7, 2025, https://pubmed.ncbi.nlm.nih.gov/38088594/
- Amgen Inc. v. Sanofi, 598 U.S. 594 (2023) | Sterne Kessler, accessed August 7, 2025, https://www.sternekessler.com/news-insights/insights/amgen-inc-v-sanofi-598-us-594-2023/
- 5 Takeaways from the U.S. Supreme Court Decision in Amgen v …, accessed August 7, 2025, https://www.procopio.com/resource/5-takeaways-scotus-amgen-sanofi
- Amgen Inc. v. Sanofi – Oyez, accessed August 7, 2025, https://www.oyez.org/cases/2022/21-757
- Clarifying the Enabling Obligation of the Patent Act: Amgen Inc. v …, accessed August 7, 2025, https://www.winston.com/en/insights-news/clarifying-the-enabling-obligation-of-the-patent-act-amgen-inc-v-sanofi
- Amgen Inc. v. Sanofi: U.S. Supreme Court Addresses Enablement for Biotechnology Inventions – Harris Beach Murtha, accessed August 7, 2025, https://www.harrisbeachmurtha.com/insights/amgen-inc-v-sanofi-u-s-supreme-court-addresses-enablement-for-biotechnology-inventions/
- Supreme Court Affirms Federal Circuit’s Amgen Decision: Patent Specification Must Enable the Full Scope of the Invention | Insights | Ropes & Gray LLP, accessed August 7, 2025, https://www.ropesgray.com/en/insights/alerts/2023/05/supreme-court-affirms-federal-circuits-amgen-decision-patent-specification-must-enable
- Summary of Amgen v. Sanofi 143 S.Ct. 1243 (2023), accessed August 7, 2025, https://www.aipla.org/docs/default-source/committee-documents/bcp-files/2023/2023-09-07-amgen-v.-sanofi-scotus.pdf?sfvrsn=94229018_5
- 2164-The Enablement Requirement – USPTO, accessed August 7, 2025, https://www.uspto.gov/web/offices/pac/mpep/s2164.html
- MPEP 2164: The Enablement Requirement, November 2024 (BitLaw), accessed August 7, 2025, https://www.bitlaw.com/source/mpep/2164.html
- Key Takeaways from the USPTO’s New Guidance on Written Description and Enablement, accessed August 7, 2025, https://www.dentons.com/en/insights/alerts/2024/january/12/key-takeaways-from-the-usptos-new-guidance-on-written-description-and-enablement
- 35 U.S. Code § 112 – Specification – Law.Cornell.Edu, accessed August 7, 2025, https://www.law.cornell.edu/uscode/text/35/112
- 2161-Three Separate Requirements for Specification Under 35 U.S.C. 112(a) or Pre-AIA 35 U.S.C. 112, First Paragraph – USPTO, accessed August 7, 2025, https://www.uspto.gov/web/offices/pac/mpep/s2161.html
- How is the written description requirement different from the enablement requirement? – BlueIron IP, accessed August 7, 2025, https://blueironip.com/ufaqs/how-is-the-written-description-requirement-different-from-the-enablement-requirement/
- Stroke of ‘Genus’: Amgen Inc. v. Sanofi – Smith Anderson, accessed August 7, 2025, https://www.smithlaw.com/newsroom/publications/stroke-of-genus-amgen-inc-v-sanofi
- PATENT ENABLEMENT AFTER AMGEN V. SANOFI – Berkeley Technology Law Journal, accessed August 7, 2025, https://btlj.org/wp-content/uploads/2025/02/0003_39-4_Moore.pdf
- Amgen v. Sanofi Ruling: Supreme Court Upholds Existing Legal …, accessed August 7, 2025, https://www.saul.com/insights/alert/amgen-v-sanofi-ruling-supreme-court-upholds-existing-legal-framework-patent
- Locke Lord QuickStudy: The Supreme Court Arguments on …, accessed August 7, 2025, https://www.troutman.com/insights/locke-lord-quickstudy-the-supreme-court-arguments-on-enablement-in-amgen-v-sanofi.html
- Amgen Inc. v. Sanofi – SCOTUSblog, accessed August 7, 2025, https://www.scotusblog.com/cases/case-files/amgen-inc-v-sanofi-2/
- Amgen Inc. v. Sanofi, No. 17-1480 (Fed. Cir. 2017) – Justia Law, accessed August 7, 2025, https://law.justia.com/cases/federal/appellate-courts/cafc/17-1480/17-1480-2017-10-05.html
- Oral Arguments – Amgen Inc. v. Sanofi – Supreme Court, accessed August 7, 2025, https://www.supremecourt.gov/oral_arguments/audio/2022/21-757
- What are the Wands factors for determining undue experimentation? – BlueIron IP, accessed August 7, 2025, https://blueironip.com/ufaqs/what-are-the-wands-factors-for-determining-undue-experimentation/
- 35 USC § 112, first paragraph and the Wands Analysis, accessed August 7, 2025, https://www.aipla.org/docs/default-source/committee-documents/bcp-files/ryucel_wa.pdf
- MPEP 2164.01(a): Undue Experimentation Factors, November 2024 (BitLaw), accessed August 7, 2025, https://www.bitlaw.com/source/mpep/2164-01-a.html
- The Death of the Genus Claim – Journal Article – Stanford Law School, accessed August 7, 2025, https://law.stanford.edu/publications/the-death-of-the-genus-claim/
- The Death of the Genus Claim1 Dmitry Karshtedt,2 Mark A. Lemley3 …, accessed August 7, 2025, https://law.stanford.edu/wp-content/uploads/2020/08/SSRN-id3668014.pdf
- Fearmongering Obscures Historical, Pro-innovation Role of Genus Claims | Hudson Institute, accessed August 7, 2025, https://www.hudson.org/intellectual-property/fearmongering-obscures-historical-pro-innovation-role-genus-claims
- The Death of the Genus Claim – Scholarly Commons – The George …, accessed August 7, 2025, https://scholarship.law.gwu.edu/cgi/viewcontent.cgi?filename=0&article=2784&context=faculty_publications&type=additional
- The U.S. Patent System, Biotechnology, and the Courts – Reaping the Benefits of Genomic and Proteomic Research – NCBI, accessed August 7, 2025, https://www.ncbi.nlm.nih.gov/books/NBK19867/
- Is the Chemical Genus Claim Really “Dead” at the Federal Circuit?: Part I – UMKC School of Law Institutional Repository, accessed August 7, 2025, https://irlaw.umkc.edu/cgi/viewcontent.cgi?article=1715&context=faculty_works
- Impact Of Amgen Inc. v. Sanofi On Patenting Antibody Based Therapeutics – American Bar Association, accessed August 7, 2025, https://www.americanbar.org/content/dam/aba/publications/Jurimetrics/winter-2024/impact-of-amgen-inc-v-sanofi-on-patenting-antibody-based-therapeutics.pdf
- After Amgen: Examining the Supreme Court’s Impact on Patents for Therapeutic Antibodies, accessed August 7, 2025, https://www.pennstatelawreview.org/print-issues/after-amgen-examining-the-supreme-courts-impact-on-patents-for-therapeutic-antibodies/
- Redefining Enablement: The Impact of Amgen v. Sanofi on Patent Law and Biotechnology, accessed August 7, 2025, https://ucipclj.org/2025/04/30/redefining-enablement-the-impact-of-amgen-v-sanofi-on-patent-law-and-biotechnology/
- Amgen v. Sanofi: Supreme Court Holds Patents Claiming Antibody Genus Invalid as Not Enabled | Congress.gov, accessed August 7, 2025, https://www.congress.gov/crs-product/LSB10971
- Amgen v. Sanofi: Seven Months In, Has Anything About Patent Enablement Changed?, accessed August 7, 2025, https://ipwatchdog.com/2024/01/09/amgen-v-sanofi-seven-months-in-has-anything-about-patent-enablement-changed/id=171703/
- Exploring Biosimilars as a Drug Patent Strategy: Navigating the …, accessed August 7, 2025, https://www.drugpatentwatch.com/blog/exploring-biosimilars-as-a-drug-patent-strategy-navigating-the-complexities-of-biologic-innovation-and-market-access/
- Amgen’s Biosimilars and Rare Disease Pipeline to Mitigate Patent Cliff Concerns – AInvest, accessed August 7, 2025, https://www.ainvest.com/news/amgen-biosimilars-rare-disease-pipeline-mitigate-patent-cliff-concerns-2507/
- Innovative Formulation Strategies for Biosimilars: Trends Focused on Buffer-Free Systems, Safety, Regulatory Alignment, and Intellectual Property Challenges – PMC – PubMed Central, accessed August 7, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC12196224/
- Pharmaceutical Patent Litigation and the Emerging Biosimilars: A Conversation with Kevin M. Nelson, JD – PMC, accessed August 7, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC5394541/
- Amgen Update on Patent Litigation Related to Celltrion Denosumab Biosimilar Products, accessed August 7, 2025, https://www.amgen.com/newsroom/company-statements/amgen-update-on-patent-litigation-related-to-celltrion-denosumab-biosimilar-products
- Biosimilars: Patent challenges and competitive effects – Morgan Lewis, accessed August 7, 2025, https://www.morganlewis.com/-/media/files/publication/outside-publication/article/lmg_mann-mahinka-biosimilarspatentcallenges_sept2014.pdf
- The Importance of Patent Enablement Across Technical Disciplines – Exponent, accessed August 7, 2025, https://www.exponent.com/article/importance-patent-enablement-across-technical-disciplines
- Amgen Inc. et al. v. Sanofi, et al.: “A New Technology, But the Legal Principle Is the Same” – Part I, accessed August 7, 2025, https://www.troutman.com/a/web/jgaPfYp9SFfxKk4Xq8a1QF/9KAKka/oct-2023_amgen-inc-et-all-v-sanofi.pdf
- New York Law Journal: The ‘Amgen v. Sanofi’ Enablement Battle and Best Practices in Light of the Federal Circuit’s 112(a) Opinion on Broad, Functional Genus Claims, accessed August 7, 2025, https://www.csglaw.com/newsroom/new-york-law-journal-the-amgen-v-sanofi-enablement-battle-and-best-practices-in-light-of-the-federal-circuits-112a-opinion-on-broad-functional-genus-claims/
- Supreme Court Affirms Federal Circuit’s Decision in Amgen v. Sanofi – Sterne Kessler, accessed August 7, 2025, https://www.sternekessler.com/news-insights/client-alerts/supreme-court-affirms-federal-circuits-decision-amgen-v-sanofi/
- Enablement Post-Amgen and New USPTO Guidelines | Womble …, accessed August 7, 2025, https://www.womblebonddickinson.com/us/insights/alerts/enablement-post-amgen-and-new-uspto-guidelines
- Patent Lists – FDA Purple Book, accessed August 7, 2025, https://purplebooksearch.fda.gov/patent-list
- Drug Patent Watch Launches Service Offering Real-Time Patent Monitoring for Pharmaceutical Companies. – GeneOnline News, accessed August 7, 2025, https://www.geneonline.com/drug-patent-watch-launches-service-offering-real-time-patent-monitoring-for-pharmaceutical-companies/
- Securing Innovation: A Comprehensive Guide to Drafting and Prosecuting Patent Applications for Biologic Drugs – DrugPatentWatch, accessed August 7, 2025, https://www.drugpatentwatch.com/blog/drafting-drug-patent-applications-for-biologic-drugs/
- Enablement, Anticipation, and Claim Strategy: Rethinking Biotech Patent Drafting Post-Agilent | Harness IP, accessed August 7, 2025, https://www.harnessip.com/blog/2025/06/26/enablement-anticipation-and-claim-strategy/
- After Amgen: Examining the Supreme Court’s Impact on Patents for Therapeutic Antibodies – Penn State Law Review, accessed August 7, 2025, https://www.pennstatelawreview.org/wp-content/uploads/2025/05/10.-Pollio_905-936.pdf
- Amgen v. Sanofi: Critical Impact on the Value of Innovative Science in Antibody Discovery, accessed August 7, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10809895/
- Are Broader Antibody Patents Possible in US Through Means-Plus-Function Claiming?, accessed August 7, 2025, https://www.cooley.com/news/insight/2025/2025-01-06-are-broader-antibody-patents-possible-in-us-through-means-plus-function-claiming
- Treatment of Antibody Claims In the U.S. After ‘Amgen v. Sanofi’ – Harness IP, accessed August 7, 2025, https://www.harnessip.com/wordpress/wp-content/uploads/2024/02/Harness_-McCarthy-and-Rakers-in-ALMs-IP-Strategist_February-2024-Issue.pdf
- Saving Genus Claims for Antibody Patents: What We Can Learn From the Foreign Jurisdictions – University of San Diego, accessed August 7, 2025, https://digital.sandiego.edu/cgi/viewcontent.cgi?article=1355&context=ilj
- USPTO Issues Post-Amgen Enablement Guidelines – Ice Miller, accessed August 7, 2025, https://www.icemiller.com/thought-leadership/uspto-issues-post-amgen-enablement-guidelines
- MPEP 2164.02: Working and Prophetic Examples, November 2024 (BitLaw), accessed August 7, 2025, https://www.bitlaw.com/source/mpep/2164-02.html
- Use of prophetic examples in patent specifications – Spruson & Ferguson, accessed August 7, 2025, https://www.spruson.com/use-of-prophetic-examples-in-patent-specifications/
- Properly Presenting Prophetic and Working Examples in a Patent Application, accessed August 7, 2025, https://www.federalregister.gov/documents/2021/07/01/2021-14034/properly-presenting-prophetic-and-working-examples-in-a-patent-application
- Prophetic Patents – UC Davis Law Review, accessed August 7, 2025, https://lawreview.law.ucdavis.edu/sites/g/files/dgvnsk15026/files/media/documents/53-2_Freilich.pdf
- Supreme Court unanimously side with Sanofi/Regeneron in PCSK9 patent fight with Amgen : r/biotech – Reddit, accessed August 7, 2025, https://www.reddit.com/r/biotech/comments/13la4to/supreme_court_unanimously_side_with/
- How Non-Product-Specific Manufacturing Patents Block Biosimilars – Duke Law Scholarship Repository, accessed August 7, 2025, https://scholarship.law.duke.edu/cgi/viewcontent.cgi?article=4128&context=dlj
- The CAFC’s Amgen v Sanofi decision spells trouble for broad functional patent claims, accessed August 7, 2025, https://www.dechert.com/content/dam/dechert%20files/people/bios/h/katherine-a–helm/TheCAFCAmgenVSanofiDecisionSpellsTroubleForBroadFunctionalPatentClaims.pdf
- Amgen v. Sanofi: Implications for ANDA and aBLA Parties | Crowell & Moring LLP, accessed August 7, 2025, https://www.crowell.com/en/insights/client-alerts/amgen-v-sanofi-implications-for-anda-and-abla-parties
- New USPTO Guidelines: After the Supreme Court’s Amgen Decision, In re Wands Factors Remain Applicable Enablement Framework | Insights | Venable LLP, accessed August 7, 2025, https://www.venable.com/insights/publications/2024/01/new-uspto-guidelines-after-the-supreme-courts
- USPTO Issues “Reasonable” Guidance on Enablement Under Amgen v. Sanofi | JD Supra, accessed August 7, 2025, https://www.jdsupra.com/legalnews/uspto-issues-reasonable-guidance-on-4409109/
- Guidelines for Assessing Enablement in Utility Applications and Patents in View of the Supreme Court Decision in Amgen Inc. et al. v. Sanofi et al. – Federal Register, accessed August 7, 2025, https://www.federalregister.gov/documents/2024/01/10/2024-00259/guidelines-for-assessing-enablement-in-utility-applications-and-patents-in-view-of-the-supreme-court
- Patent Thickets and Litigation Abuses Hinder all Biosimilars, accessed August 7, 2025, https://accessiblemeds.org/resources/press-releases/patent-thickets-and-litigation-abuses-hinder-all-biosimilars/
- PTAB statistics show interesting trends for Orange Book and biologic patents in AIA proceedings | Mintz, accessed August 7, 2025, https://www.mintz.com/insights-center/viewpoints/2231/2021-08-24-ptab-statistics-show-interesting-trends-orange-book-and
- Recent Statistics Show PTAB Invalidation Rates Continue to Climb – IPWatchdog.com, accessed August 7, 2025, https://ipwatchdog.com/2024/06/25/recent-statistics-show-ptab-invalidation-rates-continue-climb/id=178226/
- Biologics at the PTAB: Statistics and Insights into Notable Biologics Decisions | Sterne Kessler, accessed August 7, 2025, https://www.sternekessler.com/news-insights/insights/biologics-ptab-statistics-and-insights-notable-biologics-decisions/
- Trends in PTAB Trials Involving Drug and Biologic Patents – Finnegan, accessed August 7, 2025, https://www.finnegan.com/en/insights/blogs/at-the-ptab-blog/trends-in-ptab-trials-involving-drug-and-biologic-patents.html
- Amgen v. Sanofi: The Supreme Court Takes up the Enablement Requirement in the Context of Therapeutic Monoclonal Antibodies – UMKC School of Law Institutional Repository, accessed August 7, 2025, https://irlaw.umkc.edu/cgi/viewcontent.cgi?article=1731&context=faculty_works
- Post-Amgen Patent Playbook: Section 112 Under the Microscope | Law Bulletins, accessed August 7, 2025, https://www.taftlaw.com/news-events/law-bulletins/post-amgen-patent-playbook-section-112-under-the-microscope/
- Federal Circuit Follows Through on Amgen Enablement Analysis in Baxalta Inc. v. Genentech | Finnegan | Leading IP+ Law Firm, accessed August 7, 2025, https://www.finnegan.com/en/insights/blogs/prosecution-first/federal-circuit-follows-through-on-amgen-enablement-analysis-in-baxalta-inc-v-genentech.html
- Baxalta Inc. v. Genentech, Inc. (Fed. Cir. 2023) – Patent Docs, accessed August 7, 2025, https://www.patentdocs.org/2023/09/baxalta-inc-v-genentech-inc-fed-cir-2023.html
- BAXALTA INCORPORATED v. GENENTECH, INC – United States Court of Appeals for the Federal Circuit, accessed August 7, 2025, https://www.cafc.uscourts.gov/opinions-orders/22-1461.OPINION.9-20-2023_2193254.pdf
- 3510-16-P DEPARTMENT OF COMMERCE Patent and Trademark Office [Docket No. PTO-P-2023-0013] Guidelines for Assessing Enablement in – Federal Register, accessed August 7, 2025, https://public-inspection.federalregister.gov/2024-00259.pdf?1704807918
- Federal Circuit Affirms PTAB, Citing Recent SCOTUS Opinion On …, accessed August 7, 2025, https://www.lit-ip.aoshearman.com/federal-circuit-affirms-ptab-citing-recent-scotus-opinion-on-enablement
- ENABLEMENT CLAIM CONSTRUCTION (PRECEDENTIAL) LEH © 2023 O MEDYTOX, INC. V. GALDERMA S.A., Appeal No. 2022-1165 (Fed. Cir. June – Oliff PLC, accessed August 7, 2025, https://www.oliff.com/wp-content/uploads/2023/07/2022-1165-OB.pdf
- Federal Circuit Emphasizes the Non-binding Nature of the PTAB’s Preliminary Guidance Under the Pilot Program for Motions to Amend in Medytox, Inc. v. Galderma S.A. – Haug Partners, accessed August 7, 2025, https://haugpartners.com/article/preliminary-means-preliminary-federal-circuit-emphasizes-the-non-binding-nature-of-the-ptabs-preliminary-guidance-under-the-pilot-program-for-motions-to-amend-in-medytox-inc-v-galderma-s/
- Amending a Range? Better Enable It – IP Update, accessed August 7, 2025, https://www.ipupdate.com/2023/07/amending-a-range-better-enable-it/
- Medytox, Inc. v. Galderma S.A. – United States Court of Appeals for the Federal Circuit, accessed August 7, 2025, https://www.cafc.uscourts.gov/opinions-orders/22-1165.OPINION.6-27-2023_2148484.pdf
- Two Separate Analyses: Nonobviousness vs Enablement – PTAB Litigation Blog, accessed August 7, 2025, https://www.ptablitigationblog.com/two-separate-analyses-nonobviousness-vs-enablement/
- © 2024 Thomson Reuters. No claim to original U.S. Government Works. 1 2023 WL 3881360 Only the Westlaw citation is currently av – UC Berkeley Law, accessed August 7, 2025, https://www.law.berkeley.edu/wp-content/uploads/2024/01/In-re-Starrett.pdf
- Association for Accessible Medicines – Supreme Court of the United States, accessed August 7, 2025, https://www.supremecourt.gov/DocketPDF/21/21-757/254568/20230210143813184_21-757%20bsac%20Association%20for%20Accessible%20Medicines.pdf
- Writing Strong Antibody Claims: Avoiding or Addressing USPTO Rejections for Written Description and Enablement – Taft Law, accessed August 7, 2025, https://www.taftlaw.com/news-events/law-bulletins/writing-strong-antibody-claims-avoiding-or-addressing-uspto-rejections-for-written-description-and-enablement/
- The Antibody Patent Paradox – UC Berkeley Law, accessed August 7, 2025, https://www.law.berkeley.edu/wp-content/uploads/2023/12/Antibody-Patent-Paradox.pdf
- Amgen Inc. v. Sanofi | 598 U.S. ___ (2023) | Justia U.S. Supreme Court Center, accessed August 7, 2025, https://supreme.justia.com/cases/federal/us/598/21-757/
- Amgen v. Sanofi: The U.S. Supreme Court Reviews Patent Enablement | Journal of Law, Medicine & Ethics – Cambridge University Press, accessed August 7, 2025, https://www.cambridge.org/core/journals/journal-of-law-medicine-and-ethics/article/amgen-v-sanofi-the-us-supreme-court-reviews-patent-enablement/14CF691E55FF27C8A58E5F4B22DBB3D5
- Five Questions after Amgen v. Sanofi – MoFo Life Sciences, accessed August 7, 2025, https://lifesciences.mofo.com/topics/five-questions-after-amgen-v-sanofi
- The Tipping Point: Navigating the Financial and Strategic Impact of Drug Patent Expiry, accessed August 7, 2025, https://www.drugpatentwatch.com/blog/the-impact-of-patent-expiry-on-drug-prices-a-systematic-literature-review/
- The golden era of biosimilars: patent expiration wave and their impact on global healthcare, accessed August 7, 2025, https://mabxience.com/the-golden-era-of-biosimilars-patent-expiration-wave-and-their-impact-on-global-healthcare/
- Biosimilar boom ahead: Patent expirations set to reshape the global biologics market, accessed August 7, 2025, https://www.biospectrumasia.com/analysis/29/25972/biosimilar-boom-ahead-patent-expirations-set-to-reshape-the-global-biologics-market.html
- Drug Prices After Patent Expirations in High-Income Countries and Implications for Cost-Effectiveness Analyses – PMC, accessed August 7, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11329876/
- An Analysis on leveraging the patent cliff with drug sales worth USD 251 billion going off-patent and analysis of different drug – Department of Pharmaceuticals, accessed August 7, 2025, https://pharma-dept.gov.in/sites/default/files/FINAL-An%20analysis%20on%20leveraging%20the%20patent%20cliff.pdf
- Amgen Inc. v. Sanofi | Supreme Court Bulletin | US Law | LII / Legal Information Institute, accessed August 7, 2025, https://www.law.cornell.edu/supct/cert/21-757
- Amgen Inc. v. Sanofi – Ballotpedia, accessed August 7, 2025, https://ballotpedia.org/Amgen_Inc._v._Sanofi
- SCOTUS quotes Sherkow in Amgen Inc. v. Sanofi opinion | Illinois – Blogs, accessed August 7, 2025, https://blogs.illinois.edu/view/7000/1450459555
- Unpredictability In The Art: Amgen v. Sanofi In View Of “Simultaneous Conception And Reduction To Practice” | Womble Bond Dickinson, accessed August 7, 2025, https://www.womblebonddickinson.com/us/insights/articles-and-briefings/unpredictability-art-amgen-v-sanofi-view-simultaneous-conception
- Biosimilar Patent Dance: Leveraging PTAB Challenges for Strategic Advantage, accessed August 7, 2025, https://www.drugpatentwatch.com/blog/biosimilar-patent-dance-leveraging-ptab-challenges-for-strategic-advantage/
- Amgen’s Alirocumab Patents Survive Validity Challenge After Jury Trial, accessed August 7, 2025, https://www.bigmoleculewatch.com/2019/03/01/amgens-alirocumab-patents-survive-validity-challenge-after-jury-trial/
- Federal Circuit Revisits Standard for Enablement of Antibody Claims – Knobbe Martens, accessed August 7, 2025, https://www.knobbe.com/blog/federal-circuit-revisits-standard-enablement-antibody-claims/
- A Narrow Ruling on Broad Patent Claims: Baxalta v. Genentech Elongates the Amgen v. Sanofi Line of Authority, accessed August 7, 2025, https://www.cahill.com/publications/firm-memoranda/2023-10-30-a-narrow-ruling-on-broad-patent-claims-baxalta-v-genentech-elongates-the-amgen-v-sanofi-line-of-authority/_res/id=Attachments/index=0/CGR%20Memo%20-%20A%20Narrow%20Ruling%20on%20Broad%20Patent%20Claims%20Baxalta%20v.%20Genentech%20Elongates%20the%20Amgen%20v.%20Sanofi%20Line%20of%20Authority.pdf
- Enabling and Amending Medytox v. Galderma – The National Law Review, accessed August 7, 2025, https://natlawreview.com/article/amending-range-better-enable-it
- Federal Circuit Clarifies Enablement Standards: Amgen Doesn’t Apply to Anticipatory Prior Art – Patently-O, accessed August 7, 2025, https://patentlyo.com/patent/2025/06/clarifies-enablement-anticipatory.html
- 21-757 Amgen Inc. v. Sanofi (05/18/23) – Supreme Court, accessed August 7, 2025, https://www.supremecourt.gov/opinions/22pdf/21-757_k5g1.pdf
- AMGEN INC. v. SANOFI – United States Court of Appeals for the Federal Circuit, accessed August 7, 2025, https://www.cafc.uscourts.gov/opinions-orders/20-1074.opinion.2-11-2021_1731739.pdf
- 2181-Identifying and Interpreting a 35 U.S.C. 112(f) or Pre-AIA 35 U.S.C. 112, Sixth Paragraph Limitation – USPTO, accessed August 7, 2025, https://www.uspto.gov/web/offices/pac/mpep/s2181.html
- The Antibody Patent Paradox – The Yale Law Journal, accessed August 7, 2025, https://www.yalelawjournal.org/article/the-antibody-patent-paradox
- NEW DAWN FOR LIFE SCIENCES IP STRATEGY, accessed August 7, 2025, https://www.dechert.com/content/dam/dechert%20files/people/bios/h/katherine-a–helm/IAM-Special-Report-New-Dawn-for-Life-Sciences-IP-Strategy.pdf
- Implications of the BPCIA on the IP Strategies of Brand Companies and Biosimilar Developers | Sterne Kessler, accessed August 7, 2025, https://www.sternekessler.com/news-insights/insights/implications-bpcia-ip-strategies-brand-companies-and-biosimilar/
- The Impact of Amgen v. Sandoz | News & Events – Goodwin, accessed August 7, 2025, https://www.goodwinlaw.com/en/news-and-events/events/2015/03/the-impact-of-amgen-v-sandoz
- The Impact of the Federal Circuit’s Decision in Amgen vs. Sanofi on, accessed August 7, 2025, https://www.patent.net.ph/blog/the-impact-of-the-federal-circuits-decision-in-amgen-vs-sanofi-on-biologic-patent-protection
- The Success and Failures of the Patent Dance as Shown by BPCIA Litigation Cases Filed after Sandoz v. Amgen, accessed August 7, 2025, https://lawreview.law.pitt.edu/ojs/lawreview/article/view/874/534
- You Can Dance if You Want To? Initial Interpretations of the BPCIA’s Patent Dance with Sandoz and Amgen – UC Law SF Scholarship Repository, accessed August 7, 2025, https://repository.uclawsf.edu/cgi/viewcontent.cgi?article=1012&context=hastings_science_technology_law_journal
- Statistics | USPTO, accessed August 7, 2025, https://www.uspto.gov/patents/ptab/statistics
- Global Biologics Market Research Report: Forecast (2025-2030) – MarkNtel, accessed August 7, 2025, https://www.marknteladvisors.com/research-library/global-biologics-market.html
- Biologics Market Size, Share & Growth Analysis Report, 2030, accessed August 7, 2025, https://www.grandviewresearch.com/industry-analysis/biologics-market
- Biosimilars Market Products, Applications and Regulations Overview 2025: Increasing Cost of Biologic Drugs, Leading to a Greater Demand for More Affordable Options – Global Forecasts 2024-2030 – ResearchAndMarkets.com – Business Wire, accessed August 7, 2025, https://www.businesswire.com/news/home/20250311461532/en/Biosimilars-Market-Products-Applications-and-Regulations-Overview-2025-Increasing-Cost-of-Biologic-Drugs-Leading-to-a-Greater-Demand-for-More-Affordable-Options—Global-Forecasts-2024-2030—ResearchAndMarkets.com
- Global Biologics Market – Industry Trends and Forecast to 2029, accessed August 7, 2025, https://www.databridgemarketresearch.com/reports/global-biologics-market
- Amgen, Inc. v. Sanofi, Special Masters, & the Seventh Amendment Right to a Trial b – Marquette Law Scholarly Commons, accessed August 7, 2025, https://scholarship.law.marquette.edu/cgi/viewcontent.cgi?article=1053&context=ipilr
- Our search for a reliable patent intelligence solution ended with …, accessed August 7, 2025, https://www.drugpatentwatch.com/
- Recent Statistics Show PTAB Invalidation Rates Continue to Climb, accessed August 7, 2025, https://www.ipwatchdog.com/2024/06/25/recent-statistics-show-ptab-invalidation-rates-continue-climb/id=178226/