Executive Summary
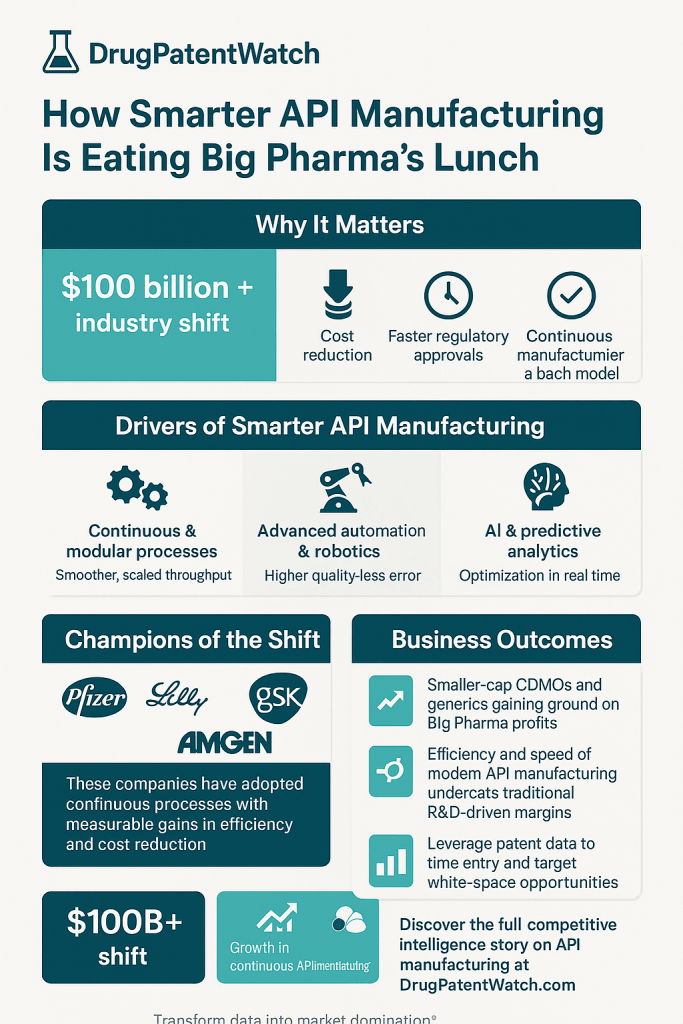
Smarter API manufacturing, powered by Industry 4.0 technologies such as the Industrial Internet of Things (IIoT), Artificial Intelligence (AI), Machine Learning (ML), and advanced analytics, and exemplified by Continuous Manufacturing (CM), is fundamentally reshaping the pharmaceutical landscape. This transformative shift provides unparalleled advantages in efficiency, quality, speed-to-market, cost reduction, and supply chain resilience, directly challenging the traditional batch-based models prevalent in Big Pharma. The cumulative effect of these advantages is creating a significant competitive divergence, where agile, tech-enabled players are poised to capture substantial market share and redefine industry leadership. This evolution effectively allows these innovators to offer superior value propositions to patients and healthcare systems, thereby disrupting established market dynamics.
Introduction: The Shifting Landscape of Pharmaceutical Manufacturing
The pharmaceutical industry, long characterized by its conservative approach and deep reliance on batch processing, is currently navigating an era of profound transformation. This shift is driven by escalating pressures from various fronts, including persistent drug shortages, inherent supply chain vulnerabilities, and increasing global demands for more affordable and personalized medicines.1 The fragility of global pharmaceutical supply chains was starkly exposed during the COVID-19 pandemic, which revealed an alarming overreliance on foreign Active Pharmaceutical Ingredient (API) manufacturing. Over 80% of APIs for U.S. medicines are currently sourced from China and India, a concentration that has led to critical shortages and significant quality control issues.2
“Smarter API manufacturing” signifies a profound paradigm shift from these traditional methods. It embraces data-driven and technology-enabled approaches to optimize every facet of production. This approach is a central component of Industry 4.0 and a subset of the broader concept of Advanced Pharmaceutical Manufacturing (APM), which aims for holistic improvements across the entire value chain.1 This transformation is not merely an incremental technological upgrade but a fundamental redefinition of pharmaceutical production, positioning agile innovators to capture significant market share and reshape industry dynamics by delivering superior value propositions.9
A critical vulnerability of Big Pharma that smarter manufacturing is actively exploiting is its traditional, globally dispersed, and often single-sourced supply chain. This long-standing model has proven highly susceptible to disruptions, leading directly to critical drug shortages and national security concerns. The current reliance on foreign API manufacturing, particularly from a limited number of regions, creates a precarious situation where geopolitical tensions, natural disasters, or trade barriers can severely impact drug availability.2 Smarter manufacturing, especially through continuous manufacturing, offers direct solutions to these vulnerabilities by enabling reshoring and diversification of production.3 This strategic advantage allows tech-enabled manufacturers to offer greater reliability and control over the supply chain, directly addressing a core weakness of traditional models.
Furthermore, the active encouragement and provision of specific guidance from influential regulatory bodies, such as the U.S. Food and Drug Administration (FDA) and the International Council for Harmonisation (ICH), for continuous manufacturing (CM) significantly de-risks its adoption for both new entrants and established players. This proactive regulatory stance fundamentally alters the risk-reward calculation for companies considering CM. Historically, regulatory hurdles have been a formidable barrier to innovation in the pharmaceutical industry, often causing hesitation in adopting new technologies.12 However, regulatory documents explicitly state that the FDA and ICH Q13 actively encourage CM, recognizing its benefits and providing detailed guidance.13 By reducing perceived regulatory uncertainty and offering a clearer path for approval, this support lowers a significant barrier to entry and adoption, making it easier for agile players to innovate and gain market share, thereby accelerating the competitive shift.
The Foundation: Understanding Smarter API Manufacturing
From Traditional Batch to Industry 4.0: A Paradigm Shift
Traditional manufacturing in the pharmaceutical sector has long been characterized by its reliance on basic machinery, extensive manual labor, and a linear, discrete batch processing approach. In this model, each stage of production must be completed before moving to the next, often sacrificing flexibility and customization for the sake of volume.7 Quality control in this conventional framework is typically reactive, known as “Quality-by-Testing” (QbT), performed at the end of each batch processing step.17 This approach inherently introduces delays and limits the ability to make real-time adjustments.
Industry 4.0, or smart manufacturing, fundamentally transforms this by integrating advanced digital technologies, including data analytics, sensors, and Artificial Intelligence (AI).6 This integration creates a highly connected, intelligent, and efficient production ecosystem. The evolution from Industry 3.0, which introduced automation and IT systems, to Industry 4.0, which connects physical assets to digital systems in a cyber-physical environment, has been driven by advancements in computing power, cloud infrastructure, sensor technology, and data analytics.6 This shift enables real-time monitoring and analysis, moving decision-making from reactive problem-solving to predictive and even prescriptive approaches.6
Core Principles of Smart Manufacturing in Pharma
Smarter manufacturing in the pharmaceutical domain is built upon several foundational principles:
- Data-Driven Decision Making: The integration of Industrial Internet of Things (IIoT) sensors and advanced analytics facilitates real-time data collection, providing profound insights into every facet of the production process. This empowers manufacturers to make informed, optimized decisions rapidly.6 By continuously monitoring key performance indicators (KPIs) and operational parameters, facilities can identify trends and anomalies that would be invisible in a traditional, less connected environment.
- Connectivity and Integration: Smart manufacturing emphasizes seamless, real-time connectivity between all digital systems, whether on-premises or cloud-based. This is often facilitated by central integration layers that enable automated workflows and synchronized data across various departments, eliminating inefficiencies caused by system fragmentation.6 This unified ecosystem ensures that information flows freely, breaking down traditional silos between different stages of production and different functional areas.
- Automation and Autonomy: A key component is the automation of workflows, supported by AI-powered quality control systems and predictive maintenance tools. This reduces human error, automates routine tasks, and significantly enhances overall operational efficiency.6 Automated systems can perform repetitive tasks with greater precision and consistency, leading to higher throughput and reduced variability.
- Flexibility and Agility: Smart manufacturing systems are designed for high adaptability, allowing manufacturers to respond swiftly to dynamic market demands, unforeseen supply chain disruptions, or the introduction of new product requirements.6 This adaptability contrasts sharply with the rigidity of traditional batch processes, which are often difficult and time-consuming to reconfigure.
The true disruptive power of smarter manufacturing extends beyond mere automation. Its capacity for predictive and prescriptive analytics allows it to anticipate and prevent issues before they occur, fundamentally enhancing efficiency and quality by shifting from reactive problem-solving to proactive optimization. While automation is a clear benefit, the ability to predict equipment wear, potential quality deviations, or impending supply chain disruptions before they materialize represents a qualitative leap.6 This proactive capability drastically minimizes unplanned downtime, reduces waste, and eliminates errors, resulting in a significantly more robust, efficient, and cost-effective process that traditional, reactive methods cannot match.
The Rise of Continuous Manufacturing: A Game Changer for APIs
Continuous Manufacturing (CM) represents a radical departure from batch processing by integrating all stages of production into a seamless, uninterrupted flow. In this innovative approach, raw materials are continuously fed into the system, transformed, and finished products are continuously removed, eliminating the distinct, interrupted phases of traditional batch production.21 This uninterrupted flow allows for a more consistent and controlled process.
CM is a central and critical component of Advanced Pharmaceutical Manufacturing (APM), a broader initiative recognized by the FDA as an emerging technology.1 It holds immense potential for improving manufacturing reliability, robustness, and ensuring timely access to quality medicines. Key operational components of CM systems include continuous flow reactors, sophisticated automated monitoring and control systems, and integrated real-time quality control mechanisms.21
The “API-first” philosophy, originating from software development, translates into a “design-first” and “Quality by Design” (QbD) approach in physical API manufacturing. This ensures that the API itself is treated as a meticulously engineered product with an optimized lifecycle and inherent quality, rather than simply a chemical output of a process. The software industry’s emphasis on designing APIs for usability and a well-managed product lifecycle finds a direct parallel in the pharmaceutical sector’s QbD principles.24 In pharmaceutical API production, this means quality is built into the process from the outset, rather than merely tested for at the end.9 A “design-first” approach to API synthesis and production involves meticulously planning, simulating, and optimizing processes
before implementation. This leads to more robust, consistent, and higher-quality APIs, reducing rework and ensuring compliance. This philosophical shift underpins the “smarter” aspect of manufacturing and differentiates it from traditional, often ad-hoc, batch optimization, contributing to the competitive advantage by producing superior products more reliably.
Enabling Technologies: The Engine of Transformation
The transformative power of smarter API manufacturing is underpinned by a suite of interconnected advanced technologies that collectively drive unprecedented levels of efficiency, precision, and control.
Industrial Internet of Things (IIoT) and Advanced Sensors
IIoT sensors and devices are instrumental in connecting machinery and equipment across the manufacturing floor, enabling real-time data collection and continuous monitoring of critical parameters such as vibration, temperature, and load levels.6 This constant stream of data forms the foundational layer for predictive maintenance, allowing manufacturers to detect early signs of equipment wear and prevent unplanned downtime, thereby optimizing overall equipment performance.6 The significance of IIoT and smart sensors is underscored by their leading market share in the tech-enabled pharmaceutical facilities market in 2024, primarily due to their crucial capability in enhancing process visibility and environmental monitoring.26
Artificial Intelligence (AI) and Machine Learning (ML) for Process Optimization
AI and ML are profoundly transforming API production, making processes more efficient, precise, and scalable.19
- Accelerated R&D and API Development: AI-powered systems can rapidly analyze vast datasets to optimize conditions for API synthesis, predict complex molecular interactions, and identify optimal reaction parameters (e.g., temperature, pressure, solvent choice). This significantly curtails the time and resources traditionally consumed in research and development.19 For example, Generative AI allowed Insilico Medicine to go from novel-target discovery to a preclinical candidate in just 18 months, spending only $2.6 million.27
- Comprehensive Process Optimization: ML models are deployed to optimize virtually every stage of API manufacturing, ranging from predictive maintenance and anomaly detection to sophisticated supply chain management and energy efficiency. AI can dynamically adjust equipment settings for peak efficiency and automate numerous routine tasks, freeing human operators for higher-value activities.18 Bosch, for instance, has leveraged ML for process optimization and automation, leading to faster production and minimized waste.18
- Enhanced Quality Control: AI and ML dramatically improve quality control through real-time monitoring of critical process parameters (e.g., temperature, pH, and pressure) and advanced anomaly detection. This allows for immediate corrective actions before a batch is compromised, ensuring consistent product quality.6 ML-powered visual inspection systems, for instance, demonstrate superior precision and speed compared to human inspectors, as seen in a refrigeration compressor manufacturer’s success with Landing AI, where the system disagreed with human experts only 5 times in 50,000 inspections and was correct in 4 of those cases.18
- Increased Productivity and Cost Reduction: Automation, driven by AI, enables systems to operate continuously with minimal human intervention, leading to shorter production cycles, higher output, and substantial savings in labor costs, energy consumption, and raw material waste.10 Google’s data centers, for example, achieved a 40% reduction in cooling energy and 15% reduction in overall power use by applying DeepMind’s machine learning, demonstrating AI’s power in energy management.28
Digital Twins and Advanced Analytics for Predictive Insights
Digital twins, which are virtual replicas of physical assets or processes, offer unprecedented capabilities for simulation, remote collaboration, and training, proving particularly valuable in decentralized or resource-constrained operational contexts.20 Their importance in achieving superior product quality with ML is widely recognized, with a significant majority (86%) of surveyed industry responders acknowledging their applicability in production operations.18
Advanced analytics platforms are designed to leverage vast amounts of data—from sensors, production lines, quality control systems, supply chains, environmental monitors, and maintenance logs—to generate actionable insights.6 Practical applications include highly accurate demand forecasting, optimizing inventory levels, efficient resource allocation, streamlining logistics and distribution channels, and pinpointing the root causes of anomalies.18 Leading pharmaceutical companies, such as Eli Lilly, are actively implementing digital twin models to enable real-time analytics and simulations, allowing them to preemptively identify potential issues, thereby reducing downtime and enhancing efficiency.10
The profound disruptive power of these enabling technologies resides in their synergistic interaction. IIoT sensors continuously generate vast amounts of real-time data, which AI/ML algorithms then process and analyze to create predictive models and automate intelligent decisions. Digital twins provide a dynamic, simulated environment for testing and optimizing these AI-driven strategies. This creates a continuous, self-improving feedback loop for relentless operational enhancement. This integrated approach fosters a “learning factory” concept, where processes constantly adapt and improve based on real-time data and predictive models, offering a significant competitive differentiator over traditional, static manufacturing processes.
Furthermore, Artificial Intelligence and advanced analytics are not merely confined to optimizing the manufacturing process itself; their influence extends to accelerating and enhancing every stage of the drug lifecycle. This includes accelerating initial discovery and development, optimizing reaction conditions for API synthesis, and enabling sophisticated supply chain management and the realization of personalized medicine.18 This broader impact indicates that smarter API manufacturing is not an isolated improvement but a critical component of a larger, AI-driven transformation spanning the
entire pharmaceutical value chain. For Big Pharma, this means that adopting AI is not just about upgrading production lines, but about integrating AI across its R&D, supply chain, and commercial operations to maintain a competitive edge and avoid being outmaneuvered by more agile, digitally native players.
The Competitive Edge: How Smarter Manufacturing “Eats Lunch”
Smarter API manufacturing, particularly through the adoption of Continuous Manufacturing (CM) and Industry 4.0 principles, offers a multifaceted competitive advantage that fundamentally challenges traditional pharmaceutical production models.
Unlocking Unprecedented Efficiency and Productivity
Automated workflows and real-time decision-making inherent in smart manufacturing lead to significantly reduced cycle times, improved throughput, and higher overall output rates.6 For instance, Pfizer successfully achieved a remarkable 50% reduction in its production cycle through the strategic adoption of automation, smart technologies, and connected worker tools.10 Continuous Manufacturing (CM) fundamentally enhances efficiency by eliminating the downtime traditionally associated with discrete batch processing, thereby substantially accelerating production timelines.21 AI-powered systems are capable of running continuously with minimal human intervention, which dramatically increases overall production capacity and consistency.19
Ensuring Superior Quality and Compliance
Smarter manufacturing inherently guarantees “Quality by Design” (QbD) through continuous, in-process monitoring, leveraging advanced Process Analytical Techniques (PAT), and enabling real-time adjustments. This results in consistently higher product quality and a significant reduction in defects.9 AI-powered anomaly detection systems are capable of identifying even subtle variations in production that could lead to quality issues, allowing for proactive and immediate corrective actions before an entire batch is compromised.19 Johnson & Johnson, for example, reported a significant reduction in error frequency by utilizing data analytics and enhanced traceability.10 The end-to-end transparency and robust data traceability facilitated by smart manufacturing systems are crucial for ensuring stringent regulatory compliance and effective risk mitigation efforts.6 The ICH Q13 guidelines actively encourage CM, specifically emphasizing the importance of data traceability and comprehensive process validation.29
Accelerating Speed to Market
Smarter manufacturing methods, particularly CM, significantly expedite both drug development and production. AI and Machine Learning (ML) accelerate initial research stages, optimize synthesis conditions, and streamline clinical trials, collectively reducing the time required for regulatory approval.10 Automation minimizes production downtime, while real-time monitoring and predictive maintenance proactively identify potential issues, leading to smoother, faster, and more reliable production flows.10 Empirical data from the FDA indicates that applications utilizing CM were approved, on average, 9 months faster and entered the market 12 months faster after regulatory submission compared to traditional batch applications.14 CM’s inherent modularity and the ability for direct transfer from R&D to production without the need for traditional scale-up (often referred to as “scale-out”) drastically cut development timelines and associated costs.22 For instance, Syntegon’s Xelum platform uses identical components across R&D and production, eliminating the need for traditional scale-up and allowing direct transfer of process parameters.30
Driving Significant Cost Reductions
Lower operational costs are a hallmark of smart manufacturing, achieved through optimized energy consumption, predictive maintenance, efficient inventory management, and a substantial reduction in rework and scrap rates.6 McKinsey estimates that advanced analytics in pharma can lead to 5-10% procurement savings, 10-20% better conversion costs, and 10-15% better cost of quality.27 Continuous processing notably reduces energy use and carbon emissions (with potential for a 20% cut in energy use) due to its reliance on significantly smaller equipment (often more than ten times smaller, requiring only a single room instead of an entire facility).31 This smaller footprint also directly translates to lower initial capital outlay and reduced running costs.31 Furthermore, CM requires less material storage, especially beneficial for highly potent materials, reducing risks and associated costs.11
Enhancing Supply Chain Resilience and Diversification
Smarter manufacturing strengthens drug supply dependability by developing new tools and technologies to address medical product shortages often caused by obsolete manufacturing and control technologies.1 CM enables rapid scaling of therapeutic production to respond more effectively to public health emergencies.1 It also creates an agile network of cost-efficient manufacturing sites that can pivot quickly to provide reserve capacity, improving supply chain resiliency.1 This approach reduces reliance on single sources and promotes the diversification of medical product sources, including APIs, excipients, and finished dose forms, by advancing domestic production and encouraging reshoring.3 The current heavy reliance of the U.S. on foreign API manufacturers, with over 80% of APIs sourced from China and India, poses significant vulnerabilities that smart manufacturing can mitigate.3
Flexibility in Production Scale and Location
Continuous manufacturing’s modularity supports adaptable and resilient manufacturing lines.31 The plant required for continuous processing is not just small; it can also be modular, making it significantly more agile than traditional batch processing. This modularity allows for easier reconfiguration of the plant in weeks rather than months or years to manufacture new products.31 This “plug-and-play” approach enables the production of more products from a single facility, meaning a plant can even be the size of a warehouse, eliminating the need for huge manufacturing sites. This flexibility allows facilities to be located nearer to customers or transport hubs, optimizing logistics and responsiveness.30
Market Growth and Trends
The global Active Pharmaceutical Ingredients (API) market is projected to grow from USD 238.38 billion in 2025 to approximately USD 405.09 billion by 2034, demonstrating a compound annual growth rate (CAGR) of 6%.33 Technavio projects a growth of USD 97.6 billion from 2025-2029, at a CAGR of over 7.1%.34 This growth is driven by several factors:
| Metric | 2025 Value (USD Billion) | 2034 Projected Value (USD Billion) | CAGR (2025-2034) |
| Global API Market Size | 238.38 | 405.09 | 6% |
| North America Market Size (2024) | 86.73 | 75.6 (by 2034) | 6.08% |
| China API Market Size (2025) | 14.48 | – | – |
| Asia Pacific Fastest Growth | – | – | 6.37% |
Data sourced from 33
Key growth drivers include the rising requirement for personalized medicine, the increasing number of groundbreaking drugs going off patent, the acceptance of quality standards like GMP and ICH guidelines, and the rising demand for oncology therapies.33 The global increase in the geriatric population and the rising prevalence of chronic and infectious diseases also contribute significantly to market expansion.35
Outsourcing of APIs has become a profitable trend, though the COVID-19 pandemic prompted companies to diversify suppliers across multiple locations for risk mitigation.35 The pharmaceutical industry is approaching a “patent cliff” by 2030, with approximately 200 molecules losing exclusivity, creating substantial opportunities for generic API manufacturers.35 North America held the largest market share in 2024 (38.36%), while Asia Pacific is projected to be the fastest-growing region (CAGR of 6.37%), driven by the expansion of pharmaceutical and contract manufacturing industries in countries like China and India.33
Intellectual Property (IP) Management
In pharmaceutical competitive intelligence, patent information plays a crucial role in assessing the intellectual property landscape. This involves monitoring competitor patent filings, analyzing patent expiration dates, and identifying potential infringement risks or licensing opportunities.36 This detailed approach enables strategic decision-making regarding product development and patent portfolio management.36 Platforms like DrugPatentWatch provide deep actionable business intelligence on small-molecule drugs and global patents, offering use cases for generic and API manufacturers to identify which drugs to develop and for branded firms seeking competitive intelligence.37
IP protection, including patents and trade secrets, promotes continuous innovation by providing financial incentives for R&D.39 It also increases economic growth by generating new jobs and boosting exports, while preventing misuse and confusion in the market.40 The introduction of the Unified Patent Court (UPC) in Europe further enhances IP protection, streamlining enforcement and accelerating product launches.39
Case Studies and Real-World Adoption
The transition to smarter API manufacturing is no longer theoretical, with several leading pharmaceutical companies actively implementing these advanced approaches. Companies such as Pfizer, Eli Lilly, GSK, and Amgen have successfully transitioned from batch to continuous processes, significantly enhancing their production efficiency, reducing costs, and meeting regulatory requirements more swiftly.5
A notable example is the Novartis-MIT Center for Continuous Manufacturing, an $85 million research project that developed and operated the first end-to-end continuous line capable of producing a pharmaceutical product.25 This breakthrough demonstrated the technical feasibility of Integrated Continuous Manufacturing (ICM), reducing the time required to transform raw materials into tablets from 200 days in traditional batch processing to just 2 days.41 This innovative platform was later commercialized by Continuus Pharmaceuticals, which is working on scaling up an end-to-end ICM pilot plant for a marketed generic drug, producing both API and tablets, with a total residence time of less than 30 hours.25
Several drug products manufactured with continuous processes have already received regulatory approval, including Vertex’s Orkambi and Symdeko/Symkevi, Johnson & Johnson’s Prezista, Eli Lilly’s Verzenio/Verzenios, and Pfizer’s Daurismo.25 While many of these are “hybrid” processes where only downstream steps are continuous, they represent significant strides. Lupin Limited has also adopted continuous manufacturing, deploying a Continuous Flow Reactor at its Ankleshwar plant in India for generic API production.41
Beyond pharma, the impact of smart manufacturing and AI is evident across various industries. A major plumbing equipment manufacturer achieved a 5.76% cost reduction and 50% faster delivery times by implementing an AI-powered system to optimize its complex supply chain.28 Google dramatically reduced cooling energy in its data centers by 40% and overall power use by 15% using DeepMind’s machine learning.28 Yates Industries, a precision machinery manufacturer, achieved 37% fewer production errors, 99.2% on-time delivery, and a 22% reduction in lead times by unifying disparate data sources into a single, intelligent system with Seraf’s Manufacturing AI Platform.28 These examples underscore the broad applicability and profound benefits of these technologies, providing a clear indication of their potential in pharmaceutical API manufacturing.
Challenges and Future Outlook
While the advantages of smarter API manufacturing are compelling, the transition from traditional batch processes presents several significant challenges and considerations for Big Pharma.
Challenges
- Initial Capital Investment and Integration Complexity: Upgrading legacy systems and deploying new technologies can be costly, requiring substantial, synchronized investments from all stakeholders, including suppliers, distributors, and equipment vendors.6 Integrating new digital systems seamlessly with existing infrastructure demands considerable expertise and careful execution.6 The existing segregated network of batch manufacturing facilities needs to be cohesively integrated, which is a major undertaking that could lead to the shutting down of plants and unit operations at distributed locations.23
- Data Overload and Cybersecurity Risks: Without a clear data strategy, manufacturers may struggle to translate raw data into actionable insights.6 Furthermore, a connected manufacturing environment increases exposure to potential cyber threats, necessitating robust safeguards.6
- Regulatory Compliance: Implementing continuous manufacturing systems requires compliance with rigorous regulatory standards, demanding thorough validation and ongoing monitoring to ensure consistent product quality and safety.21 For large molecule drugs, the robustness of CM methods is not yet well characterized due to a lack of analytic and process control methods, and there is industry hesitancy to confront regulatory uncertainties for already marketed biologic products.42
- Technical Expertise and Workforce Training: Operating and maintaining advanced continuous manufacturing systems demands a high level of technical expertise. Training and retaining skilled personnel are crucial, and investments in augmented reality (AR) and virtual reality (VR) tools may be required to facilitate rapid knowledge transfer from R&D to commercial manufacturing.21
- Operational and Material Considerations: On the shop floor, the risk of contamination and equipment breakdown is high in continuous manufacturing, requiring new technical procedures.23 The industry also needs larger vessels and continuous availability of raw materials, which necessitates re-engineering sourcing strategies.23 Tablet compression tooling, typically made of stainless steel, is not designed for continuous use, and constant force can lead to wear and tear, compromising product quality.23
- Process Understanding and Control: CM requires a high level of process understanding and control, along with robust and reliable methods for at-line process analytics and real-time monitoring, which are still under development.42 Integrating continuous downstream processing with upstream processes remains a significant hurdle in the biologics space.42
The transition to continuous manufacturing cannot be achieved by a single entity; the entire ecosystem must mature from batch to continuous manufacturing. This requires a re-engineering of all processes, including design, equipment, deployments, partners, sourcing strategy, and training.23 Quality processes must be redesigned with new procedures and equipment, as real-time release testing (RTRT) will need to be implemented across process lines for on-the-go quality feedback and approval.23 Next-generation production and quality analytics are also required for predictive insights into the continuous manufacturing process.23 Supply chain predictability and planning must be designed with newer analytical solutions for just-in-time delivery and precise inventory management, involving the development and augmentation of new supply partners.23 Information Technology (IT) will play a crucial role, as the success of CM depends on the widespread and apt use of data analytics, multivariate analysis (MVA), and RTRT to predict and improve processes. Integrated solutions with data integrity and seamless data flow from the shop floor to the top floor should be the core architecture for implementation.23
Future Trends
The future of API manufacturing and drug development is shaped by several key trends and emerging technologies 43:
- 3D Printing of APIs: Innovations in 3D-printed pharmaceuticals are expected to enable on-demand production of personalized medicines, allowing custom dosages and localized manufacturing at hospitals and pharmacies.43
- Blockchain for API Traceability: Blockchain technology is being integrated into API supply chains to enhance drug traceability, prevent counterfeit medications, and simplify regulatory transparency, ensuring quality and authenticity from production to patient.43
- Smart API Factories: The future involves fully automated smart factories equipped with AI-driven production monitoring, robotics for precise API synthesis, and real-time data analytics for predictive maintenance, aiming to meet growing demand at reduced costs.43
- Green Chemistry & Sustainable Manufacturing: Sustainability is a growing priority, with companies implementing green chemistry principles such as solvent recovery and recycling, biocatalysis, and energy-efficient manufacturing.43
- Growth in Biologics and Biosimilars: The demand for biologics and biosimilars is surging, driving API manufacturers to invest in new biomanufacturing technologies for advanced treatments.43
- Precision Medicine & Personalized APIs: Precision medicine focuses on tailoring treatments to individual patients based on genetics, lifestyle, and environment, leading to the production of small-batch APIs and customized drug formulations.43
Disruptive Potential
The disruptive potential of smarter API manufacturing is profound. It directly addresses critical national health security risks posed by the overreliance on foreign API production, which has led to drug shortages and quality issues.3 Initiatives like the Advanced Pharmaceutical Manufacturing (APM) Consortium in the U.S. aim to rapidly expand domestic production of APIs and pharmaceuticals, creating a sustainable and globally competitive APM Tech Hub.3 This effort is poised to deliver jobs and production outcomes 2-3 times faster than the current rate, accelerating the commercialization of APM product and process technologies.3 The long-term goal of reshoring 350 medicines is projected to generate $17.85 billion in economic activity, enhancing the nation’s pharmaceutical manufacturing resilience and reputation as a biopharmaceutical innovation hub.4
Conclusion
The assertion that smarter API manufacturing is “eating Big Pharma’s lunch” is substantiated by the comprehensive advantages it offers across efficiency, quality, speed-to-market, cost reduction, and supply chain resilience. The traditional batch-based model, with its inherent rigidities, reactive quality control, and vulnerable global supply chains, is increasingly challenged by the agility, precision, and proactive capabilities of Industry 4.0-driven manufacturing.
The shift from a reactive, Quality-by-Testing approach to a proactive, Quality-by-Design paradigm, enabled by the synergistic loop of IIoT, AI, ML, and digital twins, fundamentally transforms how APIs are developed and produced. This integrated technological ecosystem allows for continuous self-optimization, leading to unprecedented levels of consistency and productivity. Furthermore, the strategic adoption of continuous manufacturing, supported by favorable regulatory guidance, directly addresses Big Pharma’s historical reliance on geographically concentrated and often fragile supply chains, fostering domestic production and greater resilience.
While the initial capital investment and the need for significant ecosystem-wide maturity present hurdles, the long-term benefits in operational cost reduction, accelerated drug development, and enhanced product quality are undeniable. The market is already witnessing a clear competitive divergence, with agile, tech-enabled manufacturers demonstrating superior performance and capturing market share. For Big Pharma, the imperative is clear: embrace this transformative shift, integrate these advanced technologies across the entire drug lifecycle, and adapt business models to the new realities of intelligent, connected, and continuous production, or risk being outmaneuvered by more agile innovators. The future of pharmaceutical manufacturing is undeniably smart, continuous, and increasingly localized, promising a more robust, efficient, and patient-centric drug supply for the global community.
Works cited
- Advanced Pharmaceutical Manufacturing Concepts Are Quickly Becoming Realities, accessed July 23, 2025, https://www.pharmasalmanac.com/articles/advanced-pharmaceutical-manufacturing-concepts-are-quickly-becoming-realities
- Building Resilient Pharma Supply Chains in an Uncertain World, accessed July 23, 2025, https://www.pharmasalmanac.com/articles/building-resilient-pharma-supply-chains-in-an-uncertain-world
- Overarching Narrative – Advanced Pharmaceutical Manufacturing Tech Hub – U.S. Economic Development Administration, accessed July 23, 2025, https://www.eda.gov/sites/default/files/2024-07/APM_Tech_Hub_Overarching_Narrative.pdf
- Revitalizing U.S. Pharma: Evaluating the Economic and Social Impacts of Advanced API Manufacturing in Missouri, accessed July 23, 2025, https://olin.washu.edu/_assets/docs/research/APIIC-EconomicImpactReport.pdf
- USP Expert Discusses Balancing Drug Cost, Quality, and Access in …, accessed July 23, 2025, https://www.pharmacytimes.com/view/usp-expert-discusses-balancing-drug-cost-quality-and-access-in-a-changing-trade-landscape
- What is smart manufacturing? Benefits, examples & strategy | Blog – Alumio, accessed July 23, 2025, https://www.alumio.com/blog/what-is-smart-manufacturing
- Smart Manufacturing vs. Traditional Manufacturing: What You Need to Know, accessed July 23, 2025, https://ciotechworld.com/smart-manufacturing-vs-traditional-manufacturing-what-you-need-to-know/
- Traditional Manufacturing vs Smart Manufacturing: The Future Explained – Snic Solutions, accessed July 23, 2025, https://snicsolutions.com/blog/traditional-manufacturing-vs-smart-manufacturing
- Pharma 4.0 Smart Manufacturing: The Future of Pharma – SpendEdge, accessed July 23, 2025, https://www.spendedge.com/blogs/biotech-and-pharma-smart-manufacturing/
- 6 Benefits of Smart Manufacturing for Pharma – PharmaBoardroom, accessed July 23, 2025, https://pharmaboardroom.com/articles/6-benefits-of-smart-manufacturing-for-pharma/
- Accelerating adoption of pharmaceutical continuous … – USP, accessed July 23, 2025, https://www.usp.org/sites/default/files/usp/document/supply-chain/accelerating-continuous-manufacturing-white-paper.pdf
- Review: Continuous Manufacturing of Small Molecule Solid Oral Dosage Forms – PMC, accessed July 23, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8400279/
- Regulatory Aspects of Global Acceptance of Continuous …, accessed July 23, 2025, https://ispe.org/pharmaceutical-engineering/july-august-2021/regulatory-aspects-global-acceptance-continuous
- An FDA Self-Audit of Continuous Manufacturing for Drug Products …, accessed July 23, 2025, https://www.fda.gov/drugs/cder-small-business-industry-assistance-sbia/fda-self-audit-continuous-manufacturing-drug-products
- ICH Q13 and What Is Next for Continuous Manufacturing …, accessed July 23, 2025, https://ispe.org/pharmaceutical-engineering/ich-q13-what-next-continuous-manufacturing
- ICH guideline Q13 on continuous manufacturing of drug substances and drug products – Step 5 – EMA, accessed July 23, 2025, https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q13-continuous-manufacturing-drug-substances-and-drug-products-step-5_en.pdf
- A perspective on Quality-by-Control (QbC) in pharmaceutical continuous manufacturing – PMC, accessed July 23, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9948678/
- AI and Machine Learning in Manufacturing: Benefits, Use Cases, Trends | SPD Technology, accessed July 23, 2025, https://spd.tech/machine-learning/ai-and-ml-in-manufacturing-industry/
- AI and ML in API Production: 6 Powerful Ways to Transform – Bulkdrugs Directory, accessed July 23, 2025, https://bulkdrugsdirectory.com/ai-and-ml-in-api-production/
- AI and smart technology integration are top priorities for future labs: global survey, accessed July 23, 2025, https://www.pharmamanufacturing.com/all-articles/article/55303235/ai-and-smart-technology-integration-are-top-priorities-for-future-labs-global-survey
- Continuous Manufacturing Process and Its Impact on Pharma Manufacturing | Article, accessed July 23, 2025, https://api.drreddys.com/articles/continuous-manufacturing-process-and-its-impact-pharma-manufacturing
- Continuous Manufacturing Process Development from Lab to Market, Role of Digital Tools, accessed July 23, 2025, https://www.americanpharmaceuticalreview.com/Featured-Articles/575348-Continuous-Manufacturing-Process-Development-from-Lab-to-Market-Role-of-Digital-Tools/
- Continuous manufacturing in life sciences – a big leap – HCLTech, accessed July 23, 2025, https://www.hcltech.com/sites/default/files/documents/resources/whitepaper/files/2024/09/27/continuous-manufacturing-in-life-sciences-a-big-leap-V3.pdf
- API-First is a Strategy – What’s Your Tactical Approach? – Ambassador Labs, accessed July 23, 2025, https://www.getambassador.io/blog/api-first-strategy-tactical-approach
- Design and Commercialization of an End-to-End Continuous …, accessed July 23, 2025, https://www.continuuspharma.com/paper/design-and-commercialization-of-an-end-to-end-continuous-pharmaceutical-production-process-a-pilot-plant-case-study/
- Tech-enabled Pharmaceutical Facilities Market Size, Report by 2034, accessed July 23, 2025, https://www.precedenceresearch.com/tech-enabled-pharmaceutical-facilities-market
- 8 Use Cases For Data Analytics In Pharmaceutical Industry, accessed July 23, 2025, https://www.polestarllp.com/blog/analytics-in-pharmaceutical-companies
- Smart Manufacturing Case Studies | SerafAI Resources, accessed July 23, 2025, https://serafai.com/blog/smart-manufacturing-case-studies/
- Enhancing Pharmaceutical Quality with Continuous Manufacturing, accessed July 23, 2025, https://rzsoftware.com/continuous-manufacturing-pharmaceutical-quality/
- Continuous Manufacturing of Pharmaceuticals | Key Benefits, accessed July 23, 2025, https://www.syntegon.com/solutions/pharma/continuous-manufacturing-of-pharmaceuticals/
- 5 reasons to adopt continuous processing in pharmaceutical … – WSP, accessed July 23, 2025, https://www.wsp.com/en-gb/insights/how-continuous-processing-can-optimise-pharmaceutical-manufacturing
- Perspectives on the flexibility analysis for continuous pharmaceutical manufacturing processes – PMC – PubMed Central, accessed July 23, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC9828886/
- Active Pharmaceutical Ingredients Market Size to Hit USD 405.09 …, accessed July 23, 2025, https://www.precedenceresearch.com/active-pharmaceutical-ingredient-market
- Active Pharmaceutical Ingredients Market to Grow by USD 97.6 Billion (2025-2029), Advancing API Manufacturing in Developing Regions Boosts Revenue, AI-Powered Insights – Technavio – PR Newswire, accessed July 23, 2025, https://www.prnewswire.com/news-releases/active-pharmaceutical-ingredients-market-to-grow-by-usd-97-6-billion-2025-2029-advancing-api-manufacturing-in-developing-regions-boosts-revenue-ai-powered-insights—technavio-302341730.html
- API Market Trends | Contract Pharma, accessed July 23, 2025, https://www.contractpharma.com/exclusives/api-market-trends/
- A Complete Guide To Pharmaceutical Competitive Intelligence, accessed July 23, 2025, https://www.watchmycompetitor.com/resources/a-complete-guide-to-pharmaceutical-competitive-intelligence/
- DrugPatentWatch API, accessed July 23, 2025, https://www.drugpatentwatch.com/api.php
- DrugPatentWatch 2025 Company Profile: Valuation, Funding & Investors | PitchBook, accessed July 23, 2025, https://pitchbook.com/profiles/company/519079-87
- Unlocking intellectual property potential in European pharma, accessed July 23, 2025, https://www.europeanpharmaceuticalreview.com/article/247140/unlocking-intellectual-property-potential-in-european-pharma/
- How Does Intellectual Property Protection Work in the Pharmaceutical Industry?, accessed July 23, 2025, https://oakwoodlabs.com/how-does-intellectual-property-protection-work-in-the-pharmaceutical-industry/
- Continuous Manufacturing For Small Molecule APIs Market Report …, accessed July 23, 2025, https://www.grandviewresearch.com/industry-analysis/continuous-manufacturing-small-molecule-apis-market-report
- CONTINUOUS MANUFACTURING FOR THE MODERNIZATION OF …, accessed July 23, 2025, https://www.ncbi.nlm.nih.gov/books/NBK540224/
- API Manufacturing in 2025 l Trends l Future Innovations, accessed July 23, 2025, https://chemignition.com/blog/emerging-pharma-api-trends#:~:text=API%20manufacturing%20refers%20to%20the,biologic%20APIs%20using%20living%20organisms.