In the dynamic landscape of the pharmaceutical industry, the anticipation surrounding the launch of generic drugs is often significant. These medications, offering more affordable alternatives to their brand-name counterparts, hold the promise of increased accessibility and reduced healthcare costs. For business professionals, understanding the intricacies of when these approved generics will actually hit the market is not merely a matter of academic interest; it represents a strategic imperative. The ability to accurately predict the arrival of generic competition can unlock substantial opportunities for market analysis, investment strategies, and ultimately, market domination. This report delves into the multifaceted reasons why generic drugs, despite receiving the green light from regulatory authorities, may experience delays in their launch, and it provides actionable insights for business professionals seeking to forecast these crucial market entries.
- The Streamlined Path to Approval: Understanding the FDA’s Generic Drug Process
The journey of a generic drug to the market begins with the rigorous approval process overseen by the U.S. Food and Drug Administration (FDA). Unlike the pathway for novel medications, generic drugs follow an “abbreviated” route, primarily focused on demonstrating their similarity to an already approved brand-name drug.1
- Abbreviated New Drug Application (ANDA): An Overview
The primary mechanism for obtaining FDA approval for a generic drug is through the Abbreviated New Drug Application (ANDA). This pathway, established by the Hatch-Waxman Act of 1984, was designed to balance pharmaceutical innovation with increased access to affordable medicines.2 It allows generic manufacturers to seek approval by demonstrating that their drug is bioequivalent to a previously approved brand-name drug, known as the Reference Listed Drug (RLD).1 This approach bypasses the need for generic applicants to conduct extensive preclinical (animal) and clinical (human) studies on ingredients or dosage forms that have already been established as safe and effective for the RLD.1 This reduction in upfront research costs is a key reason why generic drugs are typically sold at significantly lower prices than their brand-name counterparts, often estimated at 80 to 85% less.16 The FDA’s Center for Drug Evaluation and Research (CDER), specifically the Office of Generic Drugs (OGD), oversees the rigorous review and ultimate approval of these ANDAs, ensuring that generic medications meet high standards of safety, efficacy, and quality.18
The reliance on existing safety and efficacy data for RLDs streamlines the approval process, making generic drug development a more cost-effective option.1 This efficiency is intended to facilitate quicker access to affordable medications for patients.18 However, while the approval pathway is abbreviated in terms of clinical trials, the FDA still mandates a thorough review of manufacturing processes and quality control measures, which can influence the overall timeline leading up to a product’s market launch. - Demonstrating Bioequivalence: The Core of Generic Approval
At the heart of the ANDA approval process lies the critical concept of bioequivalence.1 For a generic drug to be approved, its manufacturer must demonstrate to the FDA that the generic product performs in the body in the same way as the brand-name medicine.1 This includes having the same active ingredient(s) 3, the same strength 3, the same dosage form (such as a tablet or an injectable) 3, and the same route of administration (like oral, topical, or injectable).3
To prove bioequivalence, generic drug companies often conduct studies with human volunteers who take both the brand-name and the generic drug products.2 The FDA meticulously compares the data from these trials to ensure that the generic drug is safe, effective, and can be substituted for the brand-name product.2 Essentially, manufacturers must demonstrate that the right amount of the active ingredient reaches the intended site in the body at approximately the same rate and to the same extent as the brand-name drug.2 While the active ingredient must be identical, minor differences in inactive ingredients, such as colors or flavorings, are permissible as long as these variations do not affect the drug’s performance.7
The stringent bioequivalence standards are crucial for ensuring that generic drugs are therapeutically equivalent to their brand-name counterparts.4 This equivalence underpins the confidence of healthcare professionals and patients in using generic medications, which in turn leads to substantial cost savings within the healthcare system.2 However, demonstrating bioequivalence, particularly for complex drug formulations or delivery mechanisms, can be a technically challenging process that may contribute to delays in both the approval stage and the subsequent market launch. - Rigorous Review: Ensuring Safety, Efficacy, and Quality
The FDA subjects all ANDAs to a rigorous review process to guarantee that generic medicines meet the same stringent standards of safety, efficacy, and quality as brand-name drugs.1 This evaluation is conducted by a diverse team of FDA experts, including physicians, scientists, chemists, and pharmacologists.17
The review encompasses a thorough examination of the data submitted by the generic drug company, such as detailed information about the manufacturing process, the quality control measures in place, the results of stability testing to ensure the drug’s integrity over time 3, and the proposed labeling for the drug product.3 Furthermore, FDA inspectors conduct on-site visits to the manufacturing facilities of generic drug companies to verify their adherence to strict Current Good Manufacturing Practice (CGMP) regulations.3 This ensures that the manufacturer possesses the capability to consistently produce the drug to the required standards.16 Even after a generic drug receives approval and enters the market, the FDA continues its oversight by monitoring drug safety, periodically inspecting manufacturing plants, and evaluating any proposed changes to the approved generic product.6
While the ANDA process is designed to be more efficient than the NDA process, the FDA’s commitment to ensuring the safety, efficacy, and quality of generic drugs means that the review can still be a detailed and time-consuming process, sometimes involving multiple review cycles.18 If the FDA identifies any deficiencies in the submitted application or during facility inspections, it can lead to delays in the approval timeline 37, which subsequently affects when the generic drug can be launched commercially. The complexity of the specific drug product and the completeness of the submitted application also play a significant role in determining the duration of the FDA review.31 - The Patent Puzzle: How Intellectual Property Holds Up Generic Launches
Even after a generic drug successfully navigates the FDA’s rigorous approval process, its immediate launch onto the market is not always guaranteed. A significant factor that can prevent or delay the availability of approved generics is the existence of intellectual property rights, specifically patents, protecting the original brand-name drug.16
- Initial Patent Protection: Shielding Brand-Name Innovations
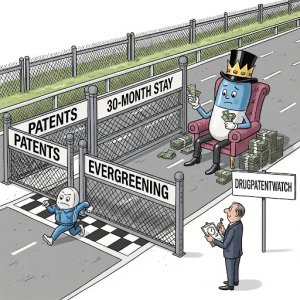
When a pharmaceutical company develops a new drug, it typically seeks patent protection from the United States Patent and Trademark Office (USPTO).16 A patent grants the innovator company exclusive rights to manufacture, use, and sell the drug for a specified period, generally 20 years from the date of filing the patent application.40 This period of market exclusivity is intended to allow the brand-name company to recoup the substantial investments made in research, development, and clinical trials required to bring the new drug to market.16 Information regarding patents that cover approved drugs is submitted by the brand-name companies to the FDA and is publicly listed in the FDA’s “Approved Drug Products with Therapeutic Equivalence Evaluations,” more commonly known as the Orange Book.2 The existence of these patents creates a legal barrier that typically prevents generic drug companies from launching their versions of the drug until the patent or all relevant patents expire.16 Consequently, even if a generic drug receives FDA approval, its launch might be delayed if the brand-name drug is still protected by an active patent. - Secondary Patents and “Evergreening” Strategies
Beyond the initial patent on the active ingredient of a drug, brand-name pharmaceutical companies often employ a strategy known as “evergreening”.41 This involves applying for and obtaining new patents on secondary aspects of the drug as the original patent approaches its expiration date. These secondary patents can cover a wide range of features, including specific formulations, particular dosage forms, methods of administration, or even new uses for the existing drug.40 The number of patents listed with the FDA for a single brand-name drug has increased significantly over the years, with the median number tripling between 1985 and 2005.55 This layering of patents, sometimes referred to as a “patent thicket” 40, can create a complex landscape of intellectual property rights surrounding a single medication. Examples of secondary patents include those protecting an extended-release formulation of a drug or a specific coating applied to a tablet.40 Even seemingly minor modifications to a drug product can potentially be patented, effectively extending the period of market exclusivity beyond the life of the original active ingredient patent.41 These “evergreening” strategies can substantially delay the market entry of generic drugs, even after the primary patent on the active ingredient has expired.40 Generic companies may be required to challenge each of these secondary patents individually, a process that can be both lengthy and expensive, or they might have to wait for each patent to expire before they can launch their generic version. This tactic allows brand-name companies to maintain their market dominance and continue to generate revenue without facing generic competition for a longer period. - Patent Litigation: The Battle for Market Entry
A generic drug company that wishes to market its product before all relevant patents listed in the Orange Book have expired has the option to submit an ANDA with a “Paragraph IV certification”.11 In this certification, the generic applicant states that the listed patent(s) are either invalid, unenforceable, or will not be infringed by their generic product.11 The submission of a Paragraph IV certification initiates a notification process, alerting the brand-name drug company and the patent holder to the generic company’s intention.13 Upon receiving this notification, if the patent holder disagrees with the generic company’s assertion of non-infringement or invalidity, they typically have 45 days to file a patent infringement lawsuit against the generic applicant.13
- Paragraph IV Challenges and the 30-Month Stay
If a patent infringement lawsuit is filed within the 45-day period following a Paragraph IV certification, it automatically triggers a 30-month stay on the FDA’s final approval of the generic drug application.13 This 30-month postponement, often simply called the “30-month stay” 13, prevents the FDA from granting final approval to the ANDA until the earliest of three conditions is met: 30 months have passed since the notification date, a court issues a decision in favor of the generic applicant (finding the patent invalid or not infringed), or the patent(s) in question expire.13 The purpose of this regulatory stay is to provide a defined timeframe for the brand-name company and the generic company to resolve their patent dispute through litigation before the generic drug can enter the market.13 Even if a generic drug receives tentative approval from the FDA, indicating that it meets all other requirements for approval, the 30-month stay will prevent the FDA from granting final approval and allowing the drug to be launched if a patent lawsuit is still ongoing.64 This 30-month stay is a significant factor that can delay the launch of an approved generic drug.13 It effectively provides the brand-name company with a period of market protection while the patent dispute is being litigated in court. However, some studies suggest that the 30-month stay period often expires well before the actual generic product launch occurs 63, indicating that other factors beyond this regulatory mechanism might ultimately determine the timing of market entry. - Impact of Court Decisions on Launch Timelines
The outcome of patent litigation plays a crucial role in determining when an approved generic drug will ultimately be launched.13 If a court rules in favor of the generic company, declaring the patent(s) invalid or not infringed, this can clear the way for the FDA to grant final approval, allowing the generic drug to be launched onto the market even before the 30-month stay period has concluded or the patent’s original expiration date.13 Conversely, a court decision upholding the validity and infringement of the brand-name company’s patent(s) can further delay or even prevent the generic launch until the patent expires. Another scenario involves a generic company choosing to engage in what is known as “launching at risk”.61 This occurs when a generic company, after receiving FDA approval (or sometimes even tentative approval), decides to launch its product while patent litigation is still in progress. This is a calculated gamble, as the generic company could be held liable for substantial damages if they are ultimately found to have infringed the brand-name company’s patent.66 Settlements between brand-name and generic drug companies are also a common occurrence and can significantly influence the timing of generic market entry.55 These settlements often involve agreements where the generic company will agree not to launch its product until a specific date, which could be before or after the patent’s full term. In some instances, these settlements have involved “pay-for-delay” agreements, where the brand-name company provides compensation to the generic company in exchange for delaying its market entry. However, such agreements have faced increasing scrutiny from regulatory bodies like the Federal Trade Commission (FTC) due to concerns about their potential anti-competitive effects and the resulting higher drug prices for consumers.55 - Exclusivity Periods: Regulatory Roadblocks for Generics
Beyond patent protection, the FDA can also grant certain periods of regulatory exclusivity to brand-name drugs, which can independently delay the launch of generic versions, even after patent protection has expired.38 These exclusivities are designed to incentivize innovation and address specific public health needs.
- New Chemical Entity (NCE) Exclusivity: Protecting Novel Drugs
The FDA can grant five years of exclusivity to the first applicant that submits an NDA for a drug containing a new active moiety (a new chemical entity) that has not been previously approved by the FDA.38 During this five-year exclusivity period, the FDA is generally prohibited from accepting for filing any ANDA that seeks approval for a generic drug containing the same active moiety, even if the patent on the brand-name drug has already expired.39 This NCE exclusivity provides a significant period of market protection for truly novel drugs, ensuring that the innovator company has at least five years without generic competition, regardless of patent status.39 Therefore, even if a generic drug receives FDA approval, its launch could be delayed if the brand-name drug benefits from ongoing NCE exclusivity. - Orphan Drug Exclusivity: Incentivizing Rare Disease Treatments
The FDA may grant seven years of orphan drug exclusivity to a drug that is approved for the treatment of a rare disease or condition, generally defined as affecting fewer than 200,000 people in the United States.38 This exclusivity prevents the FDA from approving an ANDA for the same drug for the same rare disease indication during this seven-year period, unless the generic applicant can demonstrate that their drug is clinically superior to the approved orphan drug or if the patent holder provides consent.39 This protection applies irrespective of whether the drug is also covered by patents.39 Orphan drug exclusivity serves as a strong incentive for pharmaceutical companies to invest in the development of treatments for conditions affecting small patient populations, but it can also lead to extended periods without generic competition, even after any patents have expired. - Pediatric Exclusivity: Encouraging Studies in Children
If a brand-name drug company conducts pediatric studies on its drug in response to a written request from the FDA, the company may be granted an additional six months of exclusivity.38 This pediatric exclusivity is added to any existing patent protection or other regulatory exclusivities already in place for the drug.38 This additional six-month period can effectively postpone the date when a generic drug can be launched.39 While the aim of pediatric exclusivity is to encourage the study of drugs in children and ensure appropriate labeling for pediatric use, it can also result in a further delay in the availability of generic alternatives. - Other Forms of Exclusivity and Their Impact
Several other types of regulatory exclusivity can influence the timing of generic drug launches. For instance, a three-year exclusivity can be granted for a new clinical investigation (other than bioavailability studies) that is essential for the approval of a supplement to an approved NDA, such as for a new indication, new dosage form, new strength, or new route of administration.38 Another significant exclusivity is the 180-day exclusivity granted to the first generic applicant who submits a substantially complete ANDA with a Paragraph IV certification challenging a listed patent and meets other specified requirements.11 This exclusivity period typically begins when the first generic applicant commences commercial marketing of its product or when there is a final court decision holding the relevant patent invalid, unenforceable, or not infringed, whichever occurs earlier.11 While this incentive encourages generic companies to challenge patents, it also means that other generic applicants are generally prevented from launching their products until this 180-day exclusivity period expires.13 Additionally, the FDA can grant Competitive Generic Therapy (CGT) exclusivity, which also provides 180 days of exclusivity to a generic drug applicant for a drug product where there is inadequate generic competition.38 Finally, the Generating Antibiotic Incentives Now (GAIN) exclusivity offers an additional five years of exclusivity to certain new antibiotic drugs that target specific serious or life-threatening infections.38 These various forms of regulatory exclusivity demonstrate that the path to generic market entry can be influenced by factors beyond just patent expiration, and understanding these different types and their durations is crucial for accurately predicting when a generic drug might become available. - Beyond Patents and Exclusivity: Other Factors Delaying Launch
While patent protection and regulatory exclusivities are significant factors that can delay the launch of approved generic drugs, other considerations beyond intellectual property rights also play a crucial role in determining when these medications will actually become available to patients. These factors often involve the strategic business decisions of the generic drug manufacturers themselves, as well as practical hurdles related to manufacturing and supply chains. - Business Decisions of Generic Manufacturers
Even after a generic drug receives the final stamp of approval from the FDA, the ultimate decision regarding when to launch the product rests with the application holder, which is typically the generic drug manufacturer.31 A variety of business and other considerations can influence the timeline for a generic drug to become commercially available.31
- Market Assessment and Profitability Concerns
Before launching an approved generic drug, the manufacturer will conduct a thorough assessment of the current market landscape.42 This evaluation includes analyzing the potential market size for the drug, the number of other generic competitors that are expected to enter the market (including those who may have also received approval or tentative approval), the anticipated pricing pressures in the market, and the overall potential for profitability.42 If the generic company concludes that the market is likely to be too crowded with competitors, or if the anticipated price erosion will result in unacceptably low profit margins, the company might decide to delay the launch or even forgo it altogether.42 Statistics suggest that a considerable percentage of generic drugs that receive FDA approval are never actually launched due to these economic considerations.77 For instance, it has been reported that approximately 30% of approved generics do not make it to market because the market conditions are deemed unfavorable.77 - Strategic Launch Timing and Resource Allocation
A generic drug manufacturer might also strategically delay the launch of an approved product to better align with its overall business objectives and resource allocation.42 This could involve coordinating the launch with the introduction of other products in their portfolio, effectively managing their internal resources such as sales and marketing teams, or waiting for more opportune market conditions to emerge.42 For example, a company that has received FDA approval for multiple generic drugs around the same period might choose to stagger their launches over time to optimize their supply chain, marketing efforts, and overall impact on the market. Furthermore, the anticipated timing of patent expirations for related drugs or the expected actions of competitors can also influence the strategic timing of a generic launch.41 A company might decide to wait for a key secondary patent on the brand-name drug to expire or for a competitor’s regulatory exclusivity period to end before introducing its own generic version. - Potential for Authorized Generics
The potential presence or actual launch of an authorized generic can also affect a generic drug manufacturer’s decision regarding the timing of its own product launch.69 An authorized generic is essentially the brand-name drug marketed without the brand name on its label, often by the original brand-name company itself or with its permission. These authorized generics can sometimes be priced lower than the branded product but potentially higher than a traditional generic, which can alter the competitive dynamics of the market. A generic company will need to consider the likely impact of an authorized generic on market share and pricing when deciding on the optimal timing for its own generic launch.
- Manufacturing Hurdles and Quality Control
Even after a generic drug has received FDA approval, the manufacturer must ensure that its manufacturing processes are fully scaled up to produce commercial quantities and that all necessary quality control standards are consistently met.18 Any issues encountered during this manufacturing scale-up phase, such as difficulties in sourcing raw materials, equipment malfunctions, or challenges in maintaining the required purity and potency of the drug, can lead to delays in the product’s launch timeline.16 Generic drugs are required to meet the same stringent quality standards as brand-name drugs.16 If the manufacturing facility encounters quality control problems or receives any warning letters from the FDA 78, the launch of the generic drug may be significantly delayed until these issues are fully addressed and compliance with regulatory standards is demonstrated.79 - Supply Chain Considerations and API Availability
The availability and reliability of the supply chain for a generic drug, including the active pharmaceutical ingredient (API) and other necessary excipients and packaging materials, can also have a substantial impact on the timing of its market launch.61 If there are disruptions in the supply chain, whether due to issues with the API manufacturer, logistical challenges, or geopolitical events, this can lead to delays in the production and ultimately the launch of the generic drug.71 Generic drug manufacturers often rely on third-party suppliers for the API, and any problems at the supplier level, such as manufacturing issues or regulatory compliance concerns, can have a cascading effect on the generic company’s ability to launch its product on schedule.78 Ensuring a robust and secure supply chain for all necessary components is therefore essential for a timely generic drug launch.61 - Cracking the Code: How to Tell When Generic Drugs Will Hit the Market
For business professionals, the ability to anticipate when an approved generic drug will become available can provide a significant strategic advantage. By monitoring various sources of information and understanding the factors that influence launch timelines, it is possible to develop reasonably accurate predictions. - Monitoring the FDA’s Orange Book: A Key Resource
The FDA’s Orange Book (Approved Drug Products with Therapeutic Equivalence Evaluations) is an indispensable resource for tracking patent and exclusivity information related to all approved drugs, including generics.2 It serves as the official guide to drugs that the FDA has determined to be safe and effective, along with details about any patents covering these drugs and the regulatory exclusivities they might have been granted.15
- Patent Expiration Dates and Exclusivity Information
Business professionals can utilize the Orange Book to find the expiration dates of patents that protect brand-name drugs.15 The Orange Book lists all patents submitted by the NDA holder, including the patent numbers and their corresponding expiration dates.15 Additionally, it provides information on any regulatory exclusivities granted by the FDA, such as New Chemical Entity (NCE) exclusivity, Orphan Drug Exclusivity, and Pediatric Exclusivity, along with their respective expiration dates.15 Generally, the market entry of a generic drug is anticipated to occur after the later of the patent and exclusivity expiration dates.39 However, it’s crucial to remember that ongoing patent litigation can potentially alter this timeline. The Orange Book is the primary source for understanding the intellectual property landscape surrounding a particular drug.2 By closely monitoring the expiration dates of relevant patents and regulatory exclusivities, businesses can establish the earliest possible timeframe for the launch of a generic drug. - Tracking ANDA Approvals and Tentative Approvals
The Orange Book, along with the FDA’s Drugs@FDA database 85, provides information on both approved and tentatively approved ANDAs.85 The Orange Book includes the date of approval for ANDAs that have received final approval.52 A tentative approval signifies that the FDA has concluded that the generic drug meets all the necessary requirements for approval but is currently prevented from receiving final approval due to existing patent or exclusivity rights associated with the brand-name drug.64 Tracking these approvals, especially tentative approvals, can offer an early indication of which generic companies are positioned to launch their products as soon as the legal barriers are lifted.85
- Leveraging the Drugs@FDA Database for Insights
The FDA’s Drugs@FDA database is a comprehensive tool that allows users to search for detailed information on the majority of FDA-approved prescription, generic, and over-the-counter drug products.85 It provides specifics such as approval dates, application numbers, and the names of the applicants.90 The “Drug Approval Reports by Month” feature within Drugs@FDA is particularly valuable for tracking recent approvals of generic drugs.85 By selecting “Original Abbreviated New Drug Approvals (ANDAs) by Month,” users can access a list of generic drug approvals, and “Tentative Approvals by Month” provides a similar list for drugs that have received tentative approval.87 This database is updated on a daily basis 87, offering more up-to-date information compared to the monthly updates of the Orange Book. Therefore, Drugs@FDA serves as a more granular and frequently refreshed source of data on generic drug approvals, which can be highly beneficial for business professionals seeking to anticipate near-term launch possibilities. - Following Patent Litigation and Court Filings
It is crucial to monitor any patent infringement lawsuits that are filed by brand-name companies against generic applicants who have submitted Paragraph IV certifications.47 These legal actions typically take place in U.S. district courts. The outcomes of these lawsuits can significantly influence the launch timelines of generic drugs.13 A court decision that finds the patent(s) to be invalid or not infringed can pave the way for the FDA to grant final approval, potentially leading to a generic launch even before the patent’s listed expiration date or the conclusion of a 30-month stay.13 Conversely, a court ruling that upholds the validity and infringement of the brand-name company’s patent(s) can further delay or even prevent the generic launch until the patent finally expires. Information about patent litigation can often be found in court records, legal databases, and news reports that specifically cover pharmaceutical patent disputes.47 Keeping track of the progress and outcomes of these legal proceedings is essential for gaining insights into potential delays or accelerations in the launch timelines of generic drugs. - Keeping an Eye on Pharmaceutical Company Announcements
Generic drug companies often make announcements regarding their plans for launching approved products through various channels, including press releases, statements to investors, and presentations at industry conferences.41 These announcements might occur following a favorable court decision in a patent case, after the expiration of a key patent or exclusivity period, or upon receiving final FDA approval for their generic drug. Similarly, brand-name companies may also issue announcements related to settlements they have reached with generic manufacturers, and these settlements can sometimes include specific dates on which the generic product is permitted to enter the market.61 Therefore, monitoring the news and official communications from both generic and brand-name pharmaceutical companies can provide valuable clues about the anticipated launch dates of generic drugs.41 These announcements often signal significant milestones in the journey of a generic drug toward commercial availability. - Tracking National Drug Codes (NDCs)
The National Drug Code (NDC) is a unique 10-digit, 3-segment number that is assigned to every drug product marketed in the United States.61 This code identifies the labeler (manufacturer or distributor), the specific drug product, and the package size. The appearance of an NDC for a generic drug in databases such as the National Drug Code Directory, which is maintained by the FDA 86, or in commercial drug information databases, is often a strong indication that the product has been launched or is very close to being launched into the market.61 Therefore, monitoring NDC listings can serve as a practical and reliable method for confirming whether an approved generic drug has actually entered the market and is available for distribution and sale. - Utilizing Third-Party Market Intelligence and Databases
Several third-party market intelligence firms and specialized databases focus on tracking pharmaceutical patent information, FDA approvals, litigation details, and drug launch data.39 These resources often aggregate and analyze publicly available information from various sources, providing valuable insights and predictions regarding the launch timelines of generic drugs. Subscribing to or utilizing these services can potentially save business professionals a significant amount of time and effort in collecting and interpreting the vast amount of information needed to make informed predictions about generic market entry.39 These specialized resources can offer a more comprehensive and efficient approach to tracking and forecasting when approved generic drugs are likely to become available. - The Strategic Advantage: Transforming Data into Market Domination
The ability to accurately predict when an approved generic drug will launch can provide business professionals with a significant competitive edge in the pharmaceutical industry and related sectors. By effectively utilizing the information and strategies outlined above, organizations can transform raw data into actionable intelligence, leading to market domination.
- Identifying Early Entry Opportunities
A thorough understanding of the patent landscape, including the intricacies of secondary patents and potential challenges to their validity, coupled with a clear grasp of the various regulatory exclusivity periods, can enable businesses to identify potential opportunities for early entry into the generic drug market.39 Recognizing situations where patents may be weak or where exclusivities are nearing expiration can provide a window for strategic planning and development. Furthermore, understanding the significance of being a “first-to-file” generic applicant, which can lead to a valuable 180-day period of market exclusivity 11, is crucial for companies aiming to capture a significant early market share. Proactive and diligent monitoring of patent and exclusivity information, along with a deep understanding of the regulatory landscape, can reveal potential pathways for early market entry in the generic space, offering a considerable competitive advantage. - Understanding Competitive Landscapes
Tracking the ANDA approval status and the anticipated launch timelines of competitors is essential for developing effective market entry strategies.56 Knowing which companies have received FDA approval or tentative approval for a particular generic drug and their likely launch dates allows businesses to anticipate the level of competition they will face and to plan their market entry accordingly. Assessing the number of potential generic entrants that are expected to enter the market around the same time 42 is also critical. A market with fewer anticipated competitors might present a greater opportunity for achieving a significant market share and higher profitability. A comprehensive understanding of the competitive landscape, including the number and expected launch dates of rival generic products, is therefore crucial for making informed decisions regarding pricing strategies, marketing efforts, and the overall approach to market entry. - Informing Business Decisions and Investment Strategies
Accurate insights into the timelines for generic drug launches can significantly inform a wide range of business decisions and investment strategies across the pharmaceutical industry and related sectors.41 This includes making informed choices about research and development priorities, determining the optimal timing for market entry, formulating effective pricing strategies, and planning the necessary supply chain infrastructure. Furthermore, this information can help businesses anticipate the potential price erosion that brand-name drugs will likely experience once generic competition enters the market, allowing for proactive adjustments in sales and marketing strategies.41 For investors, understanding the expected timeline for generic entry can be invaluable in making decisions about investing in brand-name versus generic drug manufacturers. Reliable predictions of generic drug launch dates provide crucial data for making sound business and investment decisions throughout the pharmaceutical value chain. This knowledge can ultimately translate into a substantial strategic and financial advantage for those who can effectively leverage it. - Illustrative Examples of Launch Delays
| Brand Name Drug | Generic Name | FDA Approval Date (First Generic) | Reason for Delay | Estimated Launch Timeframe (from approval) |
| Eliquis | Apixaban | December 23, 2019 | Patent Litigation 61 | Launched Shortly After Approval |
| Restasis | Cyclosporine Ophthalmic Emulsion | June 2022 | Formulation Trade Secrets, Complex Generic 27 | ~7 years (from tentative approval) |
| Vivitrol | Naltrexone Extended-Release Injectable Suspension | April 2023 | Complex Formulation, Bioequivalence Challenges 19 | Launched Shortly After Approval |
| Lipitor | Atorvastatin | 2006 | Patent Challenges, 180-Day Exclusivity for First Filer 11 | ~14 years (from brand launch) |
| Slynd (Brand) | Drospirenone Tablets, 4 mg | September 30, 2024 | First Generic Approval 91 | Launched Shortly After Approval |
| Gocovri (Brand) | Amantadine Extended-Release Capsules, 68.5 mg | August 26, 2024 | First Generic Approval 91 | Launched Shortly After Approval |
| Tiglutik (Brand) | Riluzole Oral Suspension, 50 mg/10 mL (5 mg/mL) | August 22, 2024 | First Generic Approval 91 | Launched Shortly After Approval |
| Lucemyra (Brand) | Lofexidine Tablets, 0.18 mg | August 20, 2024 | First Generic Approval 91 | Launched Shortly After Approval |
| Ingrezza (Brand) | Valbenazine Capsules, 40 mg, 60 mg, and 80 mg | August 7, 2024 | First Generic Approval 91 | Launched Shortly After Approval |
| Mekinist (Brand) | Trametinib Tablets, 0.5 mg and 2 mg | August 6, 2024 | First Generic Approval 91 | Launched Shortly After Approval |
| Pred Forte (Brand) | Prednisolone Acetate Ophthalmic Suspension USP, 1% | August 2, 2024 | First Generic Approval 91 | Launched Shortly After Approval |
| Pantoprazole Sodium for Injection (Brand) | Pantoprazole Sodium for Injection, 40 mg per Vial | August 1, 2024 | First Generic Approval 91 | Launched Shortly After Approval |
- Quotes from Industry Experts
“Any generic medicine must perform the same in the body as the brand-name medicine. It must be the same as a brand-name medicine in dosage, form and route of administration, safety, effectiveness, strength, and labeling… It must also meet the same high standards of quality and manufacturing as the brand-name product…” 16
“When approved, the brand drug often receives patent and other protections. The patent protection period provides the brand drug company time to recover the cost of discovering and developing the drug.” 32
“Although generic drugs offer substantial cost savings to patients, justification for their broad use should be based on assurance of the quality and substitutability of generic drug products. There are high standards for safety, efficacy, and quality in the FDA’s review of both brand and generic drugs.” 18
“Exclusivity is a period of time when a brand-name drug is protected from generic drug competition. There are different exclusivities for different situations. Exclusivity is designed to promote a balance between new drug innovation and generic drug competition.” 72
“The financial pressures in the generic market are such that it’s very hard for people to be able to raise prices, even if they needed to raise prices for quality. Their financial solvency is contingent upon meeting certain price points. Their competitor ends up coming out at a lower price point, and then they’re under pressure to be able to go down and meet it.” 83 - Illustrative Statistics on Generic Drug Launches and Delays
Approximately 90% of prescriptions filled in the United States are for generic drugs.21
Generic drugs are typically sold at a discount of 80-85% compared to their brand-name counterparts.16
The FDA approved 90 first-time generic drugs in 2023.19
Between 1985 and 2005, the median number of patents listed with the FDA for a new drug tripled, indicating the increased use of secondary patents to extend market exclusivity.55
The FDA aims to take action on Priority Review drug applications within six months, compared to ten months under standard review.17
Most brand-name drug manufacturers have a market exclusivity period of 12 to 16 years before facing generic competition.45
A study indicated that over 40% of drugs approved in 2016 had not been launched by the end of 2018.78 - Key Takeaways
The launch of an approved generic drug is a complex process influenced by a multitude of factors beyond simply receiving FDA approval. Patent protection on the brand-name drug, including the strategic use of secondary patents, can significantly delay generic entry. Various regulatory exclusivities granted by the FDA also play a crucial role in determining when generics can be launched. Furthermore, the strategic business decisions of generic manufacturers, including market assessment, profitability concerns, and resource allocation, are critical determinants of launch timing. Practical considerations such as manufacturing capabilities, quality control, and the stability of the supply chain for active pharmaceutical ingredients also contribute to the overall timeline. For business professionals seeking to gain a competitive advantage, diligently monitoring resources like the FDA’s Orange Book and Drugs@FDA database, tracking patent litigation and company announcements, and understanding the nuances of regulatory exclusivities are essential strategies for predicting when generic drugs will finally reach the market. - Frequently Asked Questions (FAQs)
- FAQ 1: What is the primary reason why an approved generic drug might not launch immediately?
- The most common reasons are the existence of active patents protecting the brand-name drug or regulatory exclusivities granted by the FDA that prevent generic market entry.
- FAQ 2: How can business professionals track the potential launch date of a generic drug?
- Professionals can monitor the FDA’s Orange Book for patent and exclusivity expiration dates, track ANDA approvals and tentative approvals in the Orange Book and Drugs@FDA database, follow patent litigation and court filings, and stay informed about announcements from pharmaceutical companies.
- FAQ 3: What is the significance of a “tentative approval” for a generic drug?
- A tentative approval from the FDA indicates that the generic drug has met all the requirements for approval but is currently blocked from final approval due to patent or exclusivity rights on the brand-name drug. This suggests a potential launch shortly after those rights expire or are resolved.
- FAQ 4: Do all generic drugs that receive FDA approval eventually get launched on the market?
- No, not all approved generic drugs are launched. Generic manufacturers may decide against launching a product due to various business reasons, such as unfavorable market conditions, intense competition, or low potential profitability.
- FAQ 5: Where can I find reliable information about the patent status and approval of generic drugs in the U.S.?
- The FDA’s Orange Book (Approved Drug Products with Therapeutic Equivalence Evaluations) and the Drugs@FDA database are the most reliable and comprehensive sources of information regarding the patent status, regulatory exclusivities, and approval of both brand-name and generic drugs in the United States.
- Conclusion
The journey of an approved generic drug to the market is a complex interplay of regulatory processes, intellectual property rights, and strategic business decisions. While the FDA’s approval signifies that a generic drug meets the necessary standards for safety, efficacy, and quality, it is not the final step before commercial availability. Understanding the potential delays caused by patents, exclusivities, and market considerations is crucial for business professionals in the pharmaceutical industry and related sectors. By diligently monitoring the various indicators and leveraging the resources available, stakeholders can gain valuable insights into the likely timelines for generic drug launches, transforming data into a significant strategic advantage in this competitive landscape. - Cited Sources
- 16
- 32
- 31
- 17
- 87
- 29
- 18
- 30
- 19
- 33
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 99
- 9
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 100
- 27
- 3
- 6
- 101
- 102
- 7
- 36
- 10
- 103
- 104
- 35
- 69
- 72
- 38
- 55
- 39
- 105
- 73
- 40
- 11
- 41
- 12
- 93
- 59
- 63
- 65
- 47
- 13
- 62
- 66
- 64
- 83
- 55
- 70
- 69
- 78
- 60
- 71
- 48
- 80
- 79
- 42
- 31
- 61
- 41
- 70
- 69
- 55
- 61
- 63
- 84
- 57
- 81
- 106
- 85
- 43
- 58
- 95
- 41
- 91
- 74
- 86
- 107
- 108
- 97
- 109
- 19
- 56
- 78
- 28
- 110 100.111 101.77 102.45 103.46 104.32 105.112 106.37 107.113 108.80 109.14 110.114 111.96 112.93 113.94 114.69 115.70 116.67 117.68 118.87 119.32 120.34 121.31 122.91 123.18 124.49 125.15 126.92 127.50 128.115 129.116 130.37 131.117 132.112 133.19 134.75 135.45 136.98 137.46 138.113 139.80 140.76 141.88 142.82 143.24 144.89 145.90 146.86 147.44 148.51 149.52 150.53 151.54
Works cited
- What is ANDA & How it Works? The Complete Guide on – Elexes, accessed May 13, 2025, https://www.elexes.com/abbreviated-new-drug-application-anda-what-it-is-how-it-works/
- Abbreviated New Drug Application – Wikipedia, accessed May 13, 2025, https://en.wikipedia.org/wiki/Abbreviated_New_Drug_Application
- The ANDA Process: A Guide to FDA Submission & Approval – Excedr, accessed May 13, 2025, https://www.excedr.com/blog/what-is-abbreviated-new-drug-application
- Complete Guide to NDA vs. ANDA: Differences, Processes, and Requirements, accessed May 13, 2025, https://synergbiopharma.com/nda-vs-anda/
- NDA vs. ANDA: A Comprehensive Guide to Key Differences, Processes, and Regulatory Requirements – Vici Health Sciences, accessed May 13, 2025, https://vicihealthsciences.com/difference-between-nda-and-anda/
- Abbreviated New Drug Application (ANDA) – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/types-applications/abbreviated-new-drug-application-anda
- Abbreviated New Drug Applications (ANDA) Explained: A Quick-Guide – The FDA Group, accessed May 13, 2025, https://www.thefdagroup.com/blog/abbreviated-new-drug-applications-anda
- Abbreviated Approval Pathways for Drug Product: 505(b)(2) or ANDA? | FDA, accessed May 13, 2025, https://www.fda.gov/drugs/cder-small-business-industry-assistance-sbia/abbreviated-approval-pathways-drug-product-505b2-or-anda
- ANDA or 505(b)(2): Choosing the Right Abbreviated Approval Pathway for Your Drug, accessed May 13, 2025, https://premier-research.com/perspectives/choosing-the-right-abbreviated-approval-pathway-for-your-drug/
- ANDA Submissions: Guidance, Process & Requirements – DocShifter, accessed May 13, 2025, https://www.docshifter.com/blog/abbreviated-new-drug-applications/
- Small Business Assistance: New 180-Day Generic Drug Exclusivity Regulations – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/cder-small-business-industry-assistance-sbia/small-business-assistance-new-180-day-generic-drug-exclusivity-regulations
- Hatch-Waxman 201 – Fish & Richardson, accessed May 13, 2025, https://www.fr.com/insights/thought-leadership/blogs/hatch-waxman-201-3/
- Small Business Assistance | 180-Day Generic Drug Exclusivity – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/cder-small-business-industry-assistance-sbia/small-business-assistance-180-day-generic-drug-exclusivity
- Timeline: Generic medicines in the US | USP, accessed May 13, 2025, https://www.usp.org/our-impact/generics/timeline-of-generics-in-us
- Orange Book 101 | The FDA’s Official Register of Drugs, accessed May 13, 2025, https://www.fr.com/insights/ip-law-essentials/orange-book-101/
- Generic Drugs – FDA – Regulations.gov, accessed May 13, 2025, https://downloads.regulations.gov/EPA-HQ-OAR-2024-0196-0003/attachment_87.pdf
- FDA Approval – Process of Approving Drugs & Medical Devices – Drugwatch.com, accessed May 13, 2025, https://www.drugwatch.com/fda/approval-process/
- How the FDA Ensures High-Quality Generic Drugs – AAFP, accessed May 13, 2025, https://www.aafp.org/pubs/afp/issues/2018/0601/p696.html
- A Review of First-Time Generic Drug Approvals – U.S. Pharmacist, accessed May 13, 2025, https://www.uspharmacist.com/article/a-review-of-firsttime-generic-drug-approvals
- Global Generic Drug Affairs | FDA, accessed May 13, 2025, https://www.fda.gov/drugs/generic-drugs/global-generic-drug-affairs
- Office of Generic Drugs – FDA, accessed May 13, 2025, https://www.fda.gov/about-fda/cder-offices-and-divisions/office-generic-drugs
- Office of Generic Drugs | Offices and Divisions – FDA, accessed May 13, 2025, https://www.fda.gov/about-fda/cder-offices-and-divisions/office-generic-drugs-offices-and-divisions
- Navigating Controlled Correspondences to Support Generic Drug Development – 02/27/2025 | FDA, accessed May 13, 2025, https://www.fda.gov/drugs/news-events-human-drugs/navigating-controlled-correspondences-support-generic-drug-development-02272025
- Generic Drugs – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/buying-using-medicine-safely/generic-drugs
- Office of Generic Drugs 2023 Annual Report – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/generic-drugs/office-generic-drugs-2023-annual-report
- What is the Office of Generic Drugs (OGD)? – Freyr Solutions, accessed May 13, 2025, https://www.freyrsolutions.com/what-is-the-office-of-generic-drugs-ogd
- Generic Drugs Forum (GDF) 2024: Regulatory Considerations to Enhance Generic Drug Access – Day 1 – YouTube, accessed May 13, 2025, https://www.youtube.com/watch?v=zyGgGF5mfnE
- Generic Drugs: Tracking US Approvals – DCAT Value Chain Insights, accessed May 13, 2025, https://www.dcatvci.org/features/generic-drugs-tracking-us-approvals/
- The Generic Drug Approval Process – YouTube, accessed May 13, 2025, https://www.youtube.com/watch?v=aIFSjUL6KFU
- Generic Drugs, Overview & Basics – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/generic-drugs/overview-basics
- What Is the Approval Process for Generic Drugs? – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/generic-drugs/what-approval-process-generic-drugs
- The Generic Drug Approval Process – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/cder-conversations/generic-drug-approval-process
- Development & Approval Process | Drugs – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/development-approval-process-drugs
- Obtaining Generic Drug Approval in the United States – DrugPatentWatch, accessed May 13, 2025, https://www.drugpatentwatch.com/blog/obtaining-generic-drug-approval-in-the-united-states/
- ANDA Submissions — Content and Format Guidance for Industry Rev. 1 – FDA, accessed May 13, 2025, https://www.fda.gov/media/128127/download
- Requirements and Resources for Approved ANDAs – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/abbreviated-new-drug-application-anda/requirements-and-resources-approved-andas
- Generic Drug Applications: FDA Should Take Additional Steps to Address Factors That May Affect Approval Rates in the First Review Cycle – GAO, accessed May 13, 2025, https://www.gao.gov/products/gao-19-565
- Frequently Asked Questions on Patents and Exclusivity – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/development-approval-process-drugs/frequently-asked-questions-patents-and-exclusivity
- Why is drug exclusivity knowledge crucial for generic drug launches? – GreyB, accessed May 13, 2025, https://www.greyb.com/blog/drug-exclusivity/
- Generic Pharmaceutical Patent and FDA Law: Navigating the Complex Landscape – DrugPatentWatch – Transform Data into Market Domination, accessed May 13, 2025, https://www.drugpatentwatch.com/blog/generic-pharmaceutical-patent-and-fda-law/
- Tracking Generic Drug Launches: A Comprehensive Guide for Pharmaceutical Professionals – DrugPatentWatch, accessed May 13, 2025, https://www.drugpatentwatch.com/blog/customer-success-will-a-generic-version-of-a-drug-launch-and-when/
- How long is the patent on a new drug before generic brands are made available?, accessed May 13, 2025, https://synapse.patsnap.com/article/how-long-is-the-patent-on-a-new-drug-before-generic-brands-are-made-available
- Generic Drugs – Availability and Patent Status, accessed May 13, 2025, https://www.drugs.com/availability/
- Patent Listing in FDA’s Orange Book – Congress.gov, accessed May 13, 2025, https://www.congress.gov/crs-product/IF12644
- Determinants of Market Exclusivity for Prescription Drugs in the United States – Commonwealth Fund, accessed May 13, 2025, https://www.commonwealthfund.org/publications/journal-article/2017/sep/determinants-market-exclusivity-prescription-drugs-united
- What’s the average time to bring a drug to market in 2022? – N-SIDE, accessed May 13, 2025, https://lifesciences.n-side.com/blog/what-is-the-average-time-to-bring-a-drug-to-market-in-2022
- Intricacies of the 30-Month Stay in Pharmaceutical Patent Cases | Articles | Finnegan, accessed May 13, 2025, https://www.finnegan.com/en/insights/articles/intricacies-of-the-30-month-stay-in-pharmaceutical-patent-cases.html
- Challenging Patents To Promote Timely Generic Drug Entry: The Second Look Act And Other Options – PORTAL, accessed May 13, 2025, https://www.portalresearch.org/blog/challenging-patents-to-promote-timely-generic-drug-entry-the-second-look-act-and-other-options
- Orange Book: What it is and how it Works – Investopedia, accessed May 13, 2025, https://www.investopedia.com/terms/o/orange-book.asp
- Approved Drug Products with Therapeutic Equivalence Evaluations | Orange Book – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/drug-approvals-and-databases/approved-drug-products-therapeutic-equivalence-evaluations-orange-book
- The FDA’s Orange Book – WAYS Pharmaceutical Services, accessed May 13, 2025, https://www.waysps.com/post/the-fda-s-orange-book
- Orange Book Data Files – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/drug-approvals-and-databases/orange-book-data-files
- Orange Book: 101 An Overview (11of27) Generic Drugs Forum 2018 – YouTube, accessed May 13, 2025, https://www.youtube.com/watch?v=Ru1xAPs8iFs
- 4 Interesting Facts About the Orange Book – Pharmacy Times, accessed May 13, 2025, https://www.pharmacytimes.com/view/4-interesting-facts-about-the-orange-book
- Strategies That Delay Market Entry of Generic Drugs – Commonwealth Fund, accessed May 13, 2025, https://www.commonwealthfund.org/publications/journal-article/2017/sep/strategies-delay-market-entry-generic-drugs
- An Effective Strategy for Generic Pharma Companies – GreyB, accessed May 13, 2025, https://www.greyb.com/blog/strategy-for-generic-pharma-companies/
- New Formulations Can Delay Generic Drug Entry, Study Finds – RAPS, accessed May 13, 2025, https://www.raps.org/news-and-articles/news-articles/2019/1/new-formulations-can-delay-generic-drug-entry-stu
- Day 181 Generic Drug Launch – A Fast and Cheap Way to Find Generic Entry Opportunities, accessed May 13, 2025, https://www.drugpatentwatch.com/blog/day-181-generic-drug-launch-a-fast-and-cheap-way-to-find-generic-entry-opportunities/
- Patent Certifications and Suitability Petitions – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/abbreviated-new-drug-application-anda/patent-certifications-and-suitability-petitions
- GENERIC DRUGS IN THE UNITED STATES: POLICIES TO ADDRESS PRICING AND COMPETITION, accessed May 13, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC6355356/
- Generic Drugs Approved but not Launched – How to Tell When Generic Drugs Will hit the Market – DrugPatentWatch, accessed May 13, 2025, https://www.drugpatentwatch.com/blog/generic-drugs-approved-but-not-launched-how-to-tell-when-generic-drugs-will-hit-the-market/
- What’s Next for Hatch-Waxman Litigation and Improper Orange Book Patent Listings?, accessed May 13, 2025, https://www.foxrothschild.com/publications/whats-next-for-hatch-waxman-litigation-and-improper-orange-book-patent-listings
- The timing of 30‐month stay expirations and generic entry: A cohort study of first generics, 2013–2020 – PMC, accessed May 13, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8504843/
- An International Guide to Patent Case Management for Judges – WIPO, accessed May 13, 2025, https://www.wipo.int/patent-judicial-guide/en/full-guide/united-states/10.13.2
- The timing of 30-month stay expirations and generic entry: A cohort study of first generics, 2013-2020 – PubMed, accessed May 13, 2025, https://pubmed.ncbi.nlm.nih.gov/33982425/
- NBER WORKING PAPER SERIES NO FREE LAUNCH: AT-RISK ENTRY BY GENERIC DRUG FIRMS Keith M. Drake Robert He Thomas McGuire Alice K. N, accessed May 13, 2025, https://www.nber.org/system/files/working_papers/w29131/w29131.pdf
- Patent Damages in “At-Risk” Generic Drug Launches: Lessons from the Federal Circuit in AstraZeneca v. Apotex – Secretariat-intl.com, accessed May 13, 2025, https://secretariat-intl.com/insights/patent-damages-in-at-risk-generic-drug-launches-lessons-from-the-federal-circuit-in-astrazeneca-v-apotex/
- Pay for Delay | Federal Trade Commission, accessed May 13, 2025, https://www.ftc.gov/news-events/topics/competition-enforcement/pay-delay
- Strategies that delay or prevent the timely availability of affordable generic drugs in the United States, accessed May 13, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC4915805/
- Strategies that delay or prevent the timely availability of affordable generic drugs in the United States, accessed May 13, 2025, https://ashpublications.org/blood/article/127/11/1398/34989/Strategies-that-delay-or-prevent-the-timely
- Addressing Generic Drug Market Challenges: Strategies for Stability and Affordability, accessed May 13, 2025, https://www.drugpatentwatch.com/blog/addressing-generic-drug-market-challenges-strategies-for-stability-and-affordability/
- Exclusivity and Generic Drugs: What Does It Mean? | FDA, accessed May 13, 2025, https://www.fda.gov/files/drugs/published/Exclusivity-and-Generic-Drugs–What-Does-It-Mean-.pdf
- Data & Market Exclusivity As Incentives in Drug Development – Scendea, accessed May 13, 2025, https://www.scendea.com/articles/blog-post-title-one-25srn-58l3m-hef63
- Preparing for New Generic Launches – Pharmacy Purchasing & Products Magazine, accessed May 13, 2025, https://www.pppmag.com/article/758
- Pathbreakers: The journey of first generics – Pharmaceutical Technology, accessed May 13, 2025, https://www.pharmaceutical-technology.com/features/pathbreakers-the-journey-of-first-generics/
- Recent trends in brand-name and generic drug competition – Duke University, accessed May 13, 2025, https://fds.duke.edu/db/attachment/2575
- Regulatory Affairs: The Generic Industry Faces External Challenges – Contract Pharma, accessed May 13, 2025, https://www.contractpharma.com/exclusives/regulatory-affairs-the-generic-industry-faces-external-challenges/
- Generics 2030 – KPMG International, accessed May 13, 2025, https://kpmg.com/kpmg-us/content/dam/kpmg/pdf/2023/generics-2030.pdf
- US Generic Pharmaceutical Industry Economic Instability – API Innovation Center, accessed May 13, 2025, https://apicenter.org/wp-content/uploads/2023/07/US-Generic-Pharmaceutical-Industry-Economic-Instability.pdf
- Authorized Generics In The US: Prevalence, Characteristics, And Timing, 2010–19, accessed May 13, 2025, https://www.healthaffairs.org/doi/10.1377/hlthaff.2022.01677
- Entry Delays and Fighting Brands: Evidence from Generics and Authorized Generics* – GitHub Pages, accessed May 13, 2025, https://rubaiyat-alam.github.io/website/pharma_ag_rubaiyat_conti.pdf
- FDA List of Authorized Generic Drugs, accessed May 13, 2025, https://www.fda.gov/drugs/abbreviated-new-drug-application-anda/fda-list-authorized-generic-drugs
- Generic Drug Development: Avoid These Common Pitfalls – DrugPatentWatch, accessed May 13, 2025, https://www.drugpatentwatch.com/blog/generic-drug-development-avoid-these-common-pitfalls/
- Congress Should Pass Legislation to End the Shenanigans That Allow Brand-Name Drug Manufacturers to Delay Generic Competition and Charge Higher Prices for Longer Periods | Association for Accessible Medicines, accessed May 13, 2025, https://accessiblemeds.org/resources/blog/end-brand-name-drug-delay-generic-competition/
- www.drugpatentwatch.com, accessed May 13, 2025, https://www.drugpatentwatch.com/blog/customer-success-will-a-generic-version-of-a-drug-launch-and-when/#:~:text=The%20Drugs%40FDA%20database%20is,as%20well%20as%20tentative%20approvals.&text=Within%20Drugs%40FDA%2C%20the%20%E2%80%9C,useful%20for%20tracking%20generic%20approvals.
- Drug Approvals and Databases | FDA, accessed May 13, 2025, https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases
- Generic Drug Development | FDA, accessed May 13, 2025, https://www.fda.gov/drugs/abbreviated-new-drug-application-anda/generic-drug-development
- Drugs | FDA, accessed May 13, 2025, https://www.fda.gov/drugs
- Drugs@FDA Data Files, accessed May 13, 2025, https://www.fda.gov/drugs/drug-approvals-and-databases/drugsfda-data-files
- Resources for Information | Approved Drugs – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/drug-approvals-and-databases/resources-information-approved-drugs
- First Generic Drug Approvals – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals
- Frequently Asked Questions on The Orange Book – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/drug-approvals-and-databases/frequently-asked-questions-orange-book
- How Patent Litigation Influences Drug Approvals and Market Entry, accessed May 13, 2025, https://patentpc.com/blog/how-patent-litigation-influences-drug-approvals-and-market-entry/
- Earlier Patent Litigation Could Accelerate US Biosimilar Market Entry, accessed May 13, 2025, https://www.centerforbiosimilars.com/view/earlier-patent-litigation-could-accelerate-us-biosimilar-market-entry
- Pinpoint Launch | Manage Generic Brands at Your Pharmacy – SupplyLogix, accessed May 13, 2025, https://www.supplylogix.com/solutions/pinpoint-suite/pinpoint-launch
- Customer Success: How do we identify generic entrants before they get FDA approval?, accessed May 13, 2025, https://www.drugpatentwatch.com/blog/customer-success-how-do-we-identify-generic-entrants-before-they-get-fda-approval/
- How to Implement a Successful Generic Drug Launch Strategy – DrugPatentWatch – Transform Data into Market Domination, accessed May 13, 2025, https://www.drugpatentwatch.com/blog/how-to-implement-a-successful-generic-drug-launch-strategy/
- US brand-name drug market exclusivity periods remain relatively unchanged over the past decade – Generics and Biosimilars Initiative, accessed May 13, 2025, https://gabionline.net/generics/research/us-brand-name-drug-market-exclusivity-periods-remain-relatively-unchanged-over-the-past-decade
- FDA’s 505(b)(2) Explained: A Guide to New Drug Applications, accessed May 13, 2025, https://www.thefdagroup.com/blog/505b2
- Food and Drug Administration – Wikipedia, accessed May 13, 2025, https://en.wikipedia.org/wiki/Food_and_Drug_Administration
- 21 CFR 314.94 — Content and format of an ANDA. – eCFR, accessed May 13, 2025, https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-314/subpart-C/section-314.94
- Abbreviated New Drug Application (ANDA) Forms and Submission Requirements – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/abbreviated-new-drug-application-anda/abbreviated-new-drug-application-anda-forms-and-submission-requirements
- ANDA Submissions — Content and Format of Abbreviated New Drug Applications – FDA, accessed May 13, 2025, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/anda-submissions-content-and-format-abbreviated-new-drug-applications
- Forms & Submission Requirements – FDA, accessed May 13, 2025, https://www.fda.gov/drugs/development-approval-process-drugs/forms-submission-requirements
- Small Business Assistance: Frequently Asked Questions for New Drug Product Exclusivity, accessed May 13, 2025, https://www.fda.gov/drugs/cder-small-business-industry-assistance-sbia/small-business-assistance-frequently-asked-questions-new-drug-product-exclusivity
- Top 10 drug launch disasters | Fierce Pharma, accessed May 13, 2025, https://www.fiercepharma.com/special-report/top-10-drug-launch-disasters
- New Drug Formulations and Their Respective Generic Entry Dates – PMC – PubMed Central, accessed May 13, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10398232/
- Dates Associated with Pharmaceutical Products – NCPDP, accessed May 13, 2025, https://www.ncpdp.org/NCPDP/media/pdf/WhitePaper/20180509-Dates-Associated-with-Pharmaceutical-Products-Paper.pdf?ext=.pdf
- Developing pharmaceutical launch leaders and company-wide capabilities at scale, accessed May 13, 2025, https://www.mckinsey.com/industries/life-sciences/our-insights/developing-pharmaceutical-launch-leaders-and-company-wide-capabilities-at-scale
- What data can tell us about drug type and launch performance – ZS, accessed May 13, 2025, https://www.zs.com/insights/what-data-tells-us-about-drug-type-and-launch-success
- Drug launches reflect overall company performance – Deloitte, accessed May 13, 2025, https://www2.deloitte.com/us/en/insights/industry/health-care/key-factors-for-successful-drug-launch.html
- FDA Approves More Generic Drugs, but Competition Still Lags | The Pew Charitable Trusts, accessed May 13, 2025, https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2019/02/fda-approves-more-generic-drugs-but-competition-still-lags
- Comparing New Prescription Drug Availability and Launch Timing in the United States and Other OECD Countries, accessed May 13, 2025, https://aspe.hhs.gov/sites/default/files/documents/430a3e61c234f06270b04414e797ad3a/new-drug-availability-launch-timing.pdf
- Drug Patent and Exclusivity Study available – USPTO, accessed May 13, 2025, https://www.uspto.gov/initiatives/fda-collaboration/drug-patent-and-exclusivity-study-available
- Generic Drugs and The Orange Book – FDA, accessed May 13, 2025, https://www.fda.gov/media/94801/download
- FDA Approval Process Longer for Generics – OncLive, accessed May 13, 2025, https://www.onclive.com/view/fda_approval_process
- DEVELOPMENT TIMELINES – Drug Development Times, What it Takes – Part 2, accessed May 13, 2025, https://drug-dev.com/development-timelines-drug-development-times-what-it-takes-part-2/