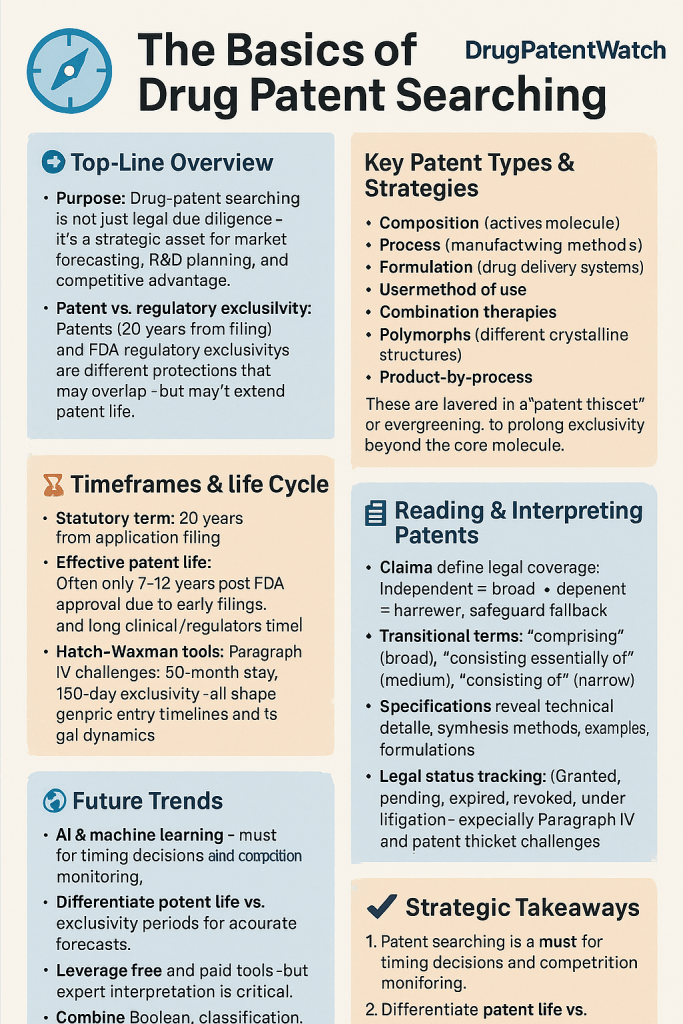
The pharmaceutical industry operates within a fiercely competitive and highly regulated environment where intellectual property, particularly drug patents, forms the bedrock of innovation and market exclusivity. Understanding the intricacies of drug patent searching is not merely a legal formality; it is a critical strategic imperative that can define a company’s success or failure. For business and pharmaceutical professionals, mastering this domain means transforming complex legal and technical data into actionable intelligence, enabling informed decisions that drive competitive advantage.
The Unseen Battleground: Why Drug Patents Define the Pharmaceutical Landscape
In the high-stakes world of drug development, patents are more than just legal documents; they are the very engine that propels innovation. They represent a fundamental economic model that allows pharmaceutical companies to undertake monumental risks and investments in the pursuit of life-saving therapies.
The Indispensable Role of Patents in Pharmaceutical Innovation
Drug patents, a vital form of intellectual property, grant their holders exclusive rights to sell a newly developed drug for a specified period, typically 20 years from the date of patent application filing.1 This exclusivity is not a luxury but a necessity. The pharmaceutical research and development (R&D) pipeline is notoriously long, expensive, and fraught with failure. Developing a new medicine can take 10-15 years and cost an average of USD 2.6 billion, with only one or two out of every 10,000 compounds successfully reaching the market. Without the prospect of exclusive market rights, the financial rationale for such high-stakes investments would largely dissipate, making it nearly impossible for companies to recoup their substantial R&D outlays and generate the profits necessary to reinvest in future discoveries.2
This protective mechanism serves as a powerful catalyst for innovation, encouraging significant investment in the development of novel drugs.7 It enables companies to maximize profits from successful treatments, which in turn attracts investors early in the development process to fund the costly clinical trials required for regulatory approval. This incentive structure is particularly vital for areas like orphan diseases, which affect fewer than 200,000 people annually in the U.S..2 For such conditions, additional exclusivity periods and greater flexibility in pricing are often granted, further encouraging drug companies to invest in treatments for smaller patient populations that might otherwise be less profitable. The very existence of these exclusive rights makes the massive R&D investments economically viable, forming the foundation of the current pharmaceutical innovation model.
The patent system operates on a fundamental “bargain”: in exchange for government-backed protection, the inventor must fully disclose enough detail about the invention so that others can understand and replicate it once the patent term expires. This public disclosure, while eventually leading to generic competition, simultaneously fuels further innovation by adding to the collective body of technical knowledge. However, this dual role of patents—as both catalysts for innovation and temporary barriers to generic competition —highlights a fundamental tension in public health policy: how to foster breakthrough discoveries while ensuring timely access to affordable medicines. This inherent conflict is a recurring theme that shapes the entire pharmaceutical landscape.
Navigating the Dual Pillars: Patents vs. Regulatory Exclusivities
A common point of confusion in the pharmaceutical industry is the distinction between patents and regulatory exclusivities. While both provide market protection, they are distinct legal instruments that operate under different authorities and rules.
Patents are property rights issued by the United States Patent and Trademark Office (USPTO) to an inventor, granting the right to exclude others from making, using, offering for sale, or selling the invention for a limited time, generally 20 years from the date of patent application filing.1 Regulatory exclusivity, conversely, refers to exclusive marketing rights granted by the Food and Drug Administration (FDA) upon the approval of a drug. This prevents the submission or effective approval of generic applications for a specified period.4
A crucial point to grasp is that exclusivity periods are not added to the patent life. They are separate legal mechanisms that can run concurrently with a patent or independently. This distinction is vital for accurate market forecasting and competitive analysis. The primary purpose of exclusivity is to balance new drug innovation with generic drug competition. Exclusivity is granted upon approval if statutory requirements are met, with durations varying based on the type of exclusivity, such as New Chemical Exclusivity, Orphan Drug Exclusivity, or Pediatric Exclusivity.4
The fact that patents and exclusivities may or may not run concurrently and may or may not encompass the same claims indicates a deliberate strategic layering of protection by innovator companies. This is not an accidental outcome; it is a calculated effort to maximize market exclusivity. The distinction and potential non-concurrency of these protections mean that generic manufacturers face a more complex landscape than simply waiting for a single patent to expire. They must navigate both patent expiry dates and various exclusivity periods, which significantly impacts their market entry timelines and strategies. This necessitates sophisticated intelligence to determine the true “loss of exclusivity” date for a drug.
Table 1: Patents vs. Regulatory Exclusivities (US)
| Feature | Patents (US) | Regulatory Exclusivities (US) |
| :— | :— |:— | | Scope of Protection | Protects the invention itself (e.g., composition, method of use, formulation, polymorph) | Grants exclusive marketing rights for the approved product/indication, preventing FDA approval of competitor drugs | | Primary Purpose | Incentivize R&D and recoup investment by granting a temporary monopoly | Balance new drug innovation with generic competition | | Standard Duration | 20 years from filing date | Varies by type (e.g., NCE: 5 years, Orphan: 7 years, Biologics: 12 years) | | Start Date | Can be issued or expire at any time during development, regardless of approval status | Granted only upon approval of a drug product if statutory requirements are met | | Challengeability | Subject to challenge (e.g., validity, infringement) | Cannot be legally challenged | | Link to FDA Orange Book | Listed in Orange Book | Listed in Orange Book |
Decoding the DNA of Drug Patents: Foundational Concepts
To effectively search and interpret drug patents, one must first grasp their fundamental building blocks. This section demystifies the core concepts, from the definition of a drug patent to the various types of inventions they protect.
What Exactly is a Drug Patent?
A drug patent is a legal document that grants an inventor the sole right to market their invention for a specified period, typically 20 years from the date of the patent application filing.1 It functions as a property right issued by the USPTO, allowing the patent holder to exclude others from making, using, offering for sale, or selling the invention throughout the United States or importing it into the country for a limited time.
Like any other invention, pharmaceutical innovations must meet stringent criteria to be patentable: they must be new (novel), useful, non-obvious, and sufficiently described.9 The non-obviousness requirement ensures that the invention is not merely an incremental or predictable development that would be apparent to someone with ordinary skill in the relevant technical field.
While the statutory patent term is generally 20 years from filing 1, the commercially impactful period after a drug receives regulatory approval is often considerably shorter. Companies frequently apply for patents long before clinical trials commence, to attract potential investors and secure funding needed for advancing the drug to later stages of clinical development.2 This early filing, while a business necessity, means that a significant portion of the 20-year patent term is consumed during the lengthy R&D and regulatory review processes. As a result, the effective patent period after a drug has received final approval is often around seven to twelve years.3 This substantial reduction in effective market exclusivity is a critical factor for business planning, driving companies to explore strategies like evergreening and patent term extensions to prolong their period of market dominance.
The Anatomy of a Patent: Understanding Key Components
A patent document is a complex legal and technical blueprint of an invention. Understanding its key components is essential for extracting meaningful intelligence.
The claims are arguably the most critical part of a patent. These are the legal statements that precisely define the scope of an invention’s protection, outlining what is new and unique and establishing the boundaries of intellectual property. They are typically written as a single, heavily punctuated sentence and appear towards the end of the issued patent or patent application. The precise wording of these claims is paramount, as they form the primary legal battleground for patent infringement and validity disputes.
- Independent Claims: These claims stand on their own, not referring to any other claim, and provide the broadest possible protection for the core invention.16 For example, a claim might state: “A composition, comprising a solvent, and a metal salt dissolved in the solvent”.
- Dependent Claims: These claims refer back to and incorporate the limitations of an independent claim, adding further features or limitations to narrow the scope of protection.16 They serve as more specific contingency positions, providing narrower protection that might be upheld even if a broader independent claim is challenged or invalidated. For instance, following the example above, a dependent claim could be: “The composition of claim 1, further comprising a pH-modifying substrate.” Dependent claims are not merely narrower fallback positions; they can also clarify and, in some instances, expand the understanding of the broader claim’s scope, as demonstrated in legal precedents where dependent claims helped interpret the breadth of an independent claim.
The choice of transitional phrases within claims (e.g., “comprising,” “consisting essentially of,” “consisting of”) significantly dictates the claim’s scope. “Comprising” is an open-ended term, meaning the invention includes the recited elements but may also include others, making it the broadest. “Consisting essentially of” represents a middle ground, indicating the invention includes the recited elements and is open to others that do not materially affect the novel properties of the claimed invention. This phrase proved critical in the Eye Therapies case, where its interpretation, informed by the patent’s prosecution history, led to a finding that brimonidine was the sole active ingredient. “Consisting of” is the most restrictive, meaning the invention includes only the recited elements.
Claim interpretation typically accords claims their plain and ordinary meaning, but an inventor can act as their own lexicographer to define terms within the patent. The prosecution history, which documents the dialogue between the applicant and the patent office, can profoundly influence this interpretation, especially when atypical meanings are asserted or when the scope of claims is ambiguous. The precise choice of transitional phrases directly impacts the scope of protection and can be the deciding factor in patent infringement litigation. Drafting strong patent claims for drug inventions is a meticulous process, akin to “sculpting a masterpiece,” requiring a deep understanding of prior art, legal precepts, and strategic foresight to create a “resilient and protective shield” for the drug invention.
The specification or detailed description provides a thorough explanation of the invention, including examples of how it can be used, and often includes illustrations. This section is where the “patent bargain” of public disclosure is fulfilled, ensuring that enough detail is provided for others to understand and replicate the invention once the patent term expires. It typically covers the chemical structure, synthesis methods, therapeutic uses, formulation details, and pharmacological properties of the drug.
Many patents describe the synthesis methods used to create the drug. These descriptions can range from general overviews to detailed, step-by-step procedures, including information on starting materials, reaction conditions, purification techniques, and yield optimization. The level of detail in the patent specification regarding synthesis methods directly impacts the ease with which generic manufacturers can “design around” the patent or develop their own non-infringing processes. A thorough specification, while fulfilling disclosure requirements, can inadvertently provide a roadmap for competitors.
The specification may also contain experimental data, presented as “working examples” (describing actual performed work or achieved results) or “prophetic examples” (describing predicted or simulated results). For pharmaceutical inventions, some data demonstrating a substantial likelihood of the invention working as a human pharmaceutical is generally required. Strategic analysis of the specification helps in identifying active ingredients, potential modifications, and planning synthesis routes. It also reveals the exact scope of protection, potential vulnerabilities, and ownership information. The requirement for the specification to be “sufficiently described” or to “fully disclose enough detail… so that others can understand and replicate it” is known as the “enablement” requirement in patent law. This ensures the public benefit of disclosure is met, even if it aids future competitors.
Drawings serve as visual aids that clarify complex processes and structures. Finally, a concise abstract provides a summary of the invention.27
Types of Pharmaceutical Patents: Beyond the Molecule
Pharmaceutical companies often pursue a multi-layered patent strategy, securing protection for various aspects of a drug to create a comprehensive “web of protection”. This approach involves different types of patents beyond just the active molecule.
- Composition of Matter Patents: These are considered the most valuable and fundamental patents in the pharmaceutical industry. They protect the active pharmaceutical ingredient (API) itself, ensuring that no other company can manufacture, use, or sell the patented chemical entity without permission during the patent term.8
- Process Patents: These protect the method of producing a specific pharmaceutical product, focusing on innovative manufacturing procedures or chemical processes developed by companies.6 They are vital for enhancing efficiency and effectiveness in production.
- Use Patents (Method of Use): These protect specific uses of a known product, including newly discovered therapeutic uses for existing drugs. This encourages drug repurposing and optimizing existing pharmaceutical products.6
- Formulation Patents: These protect the unique combination of ingredients in a drug, including special carriers, delivery mechanisms, or packaging that optimizes the drug’s performance.6 Examples include time-release capsules or novel delivery systems that target drugs directly to affected organs.8
- Combination Patents: These cater to drugs that combine multiple active ingredients to create a new therapy. DrugPatentWatch analyses highlight the importance of patenting the combination as a whole, rather than focusing solely on individual components, to strengthen the patent position and discourage replication.
- Polymorph Patents: These protect different crystalline forms of a drug substance, which can exhibit distinct physical properties such as solubility or stability.
- Product-by-Process Patents: This type of claim protects a chemical or other process used to manufacture the drug, and importantly, it also confers protection against the importation of a product made by that patented process.
The existence of multiple patent types beyond the active ingredient (e.g., formulation, use, process, polymorphs) 6 directly enables “evergreening” strategies. This is a deliberate approach by innovator companies to extend the lifetime of their patent monopolies, often by surrounding an original inventive patent with numerous additional patents that may have limited or no inventiveness.7 This practice aims to prolong monopoly periods and delay generic drug entry.7 While some secondary patents protect “genuine follow-on innovation,” distinguishing this from strategic use to extend monopolies is often “very difficult”. This raises important questions about the true innovative value of some later-stage patents versus their primary role in market protection, highlighting a major point of controversy and policy debate within the pharmaceutical industry.
The Clock is Ticking: Patent Duration and Key Dates
Understanding the lifecycle of a patent and its associated key dates is fundamental for strategic planning in the pharmaceutical sector.
The standard patent term in the United States is generally 20 years from the date of the patent application filing.1 However, this statutory term often differs significantly from the
effective patent life. Due to the lengthy R&D and regulatory approval processes inherent in drug development, the effective patent period after a drug receives FDA approval is considerably shorter, typically ranging from 7 to 12 years.3 This disparity arises because companies often file patents very early in the development process to secure priority and attract necessary investment. This business imperative of early patenting to secure funding directly leads to a reduced commercially valuable period of exclusivity post-approval, creating a fundamental tension for innovators.
Several key dates are crucial for tracking a drug patent:
- Priority Date: This is the earliest date on which an inventor can establish a date of invention. It is critically important because it determines what references can be asserted as prior art against a patent application during its examination. In a “race to the patent office” system, the inventor with the earliest priority date generally wins the rights to the patent. The priority date may be the same as the initial filing date, or it can be claimed from an earlier-filed application, such as a provisional patent application.
- Filing Date: This is the date the patent application is formally submitted to the patent office. This date marks the beginning of the 20-year patent term.1
- Publication Date: Patent applications are typically published 18 months from their priority date.34 This public disclosure transforms a regulatory requirement into a powerful strategic intelligence tool. It provides a valuable intelligence opportunity, serving as an “early warning system” for competitive threats by allowing companies to systematically track new filings and gain insights into competitor R&D pipelines years before products reach the market.
- Grant Date: This is the date the patent office officially grants the patent. It is distinct from the priority date.
- Expiration Date: This is the date the patent protection ends, opening the door for generic competition.2
The Regulatory Compass: FDA’s Role and Market Exclusivities
The pharmaceutical landscape in the United States is heavily influenced by the Food and Drug Administration (FDA) and its regulatory exclusivities. These mechanisms interact intricately with patents to shape market dynamics, with a particular focus on the landmark Hatch-Waxman Act.
The Orange Book and Purple Book: Your Essential Guides
For anyone seeking to determine the patent and exclusivity status of a drug in the United States, two primary governmental resources are indispensable: the FDA’s Orange Book and the U.S. Patent and Trademark Office (USPTO) databases.
The FDA’s Orange Book, officially known as “Approved Drug Products with Therapeutic Equivalence Evaluations,” stands as the authoritative U.S. database listing all FDA-approved non-biologic (small-molecule) drugs.4 It meticulously links approved drug products to their associated patents and regulatory exclusivity information.4 Patents eligible for Orange Book listing generally cover the drug substance (active ingredient), the drug product (formulation and composition), or the approved method of use.12 Crucially, patents claiming the process or manufacture of the drug substance, its packaging, or metabolites or intermediates are
not eligible for listing.12 The Orange Book is not merely a list; it serves as a de facto roadmap for generic manufacturers. By analyzing patent expiration dates and regulatory exclusivity periods within it, companies can identify optimal timing for market entry with generic or biosimilar products. This precise timing is critical, as entering too early risks patent infringement litigation, while entering too late means missing the first-to-file advantage that can secure a period of market exclusivity for generic manufacturers.
For biological products, such as vaccines, blood components, and monoclonal antibodies, the FDA maintains a separate publication known as the Purple Book.12 This database lists FDA-approved biological drugs and provides valuable insights into their intellectual property landscape, including patent details.
These books are critical resources for a wide range of stakeholders, including healthcare providers, pharmacists, and drug manufacturers. For drug manufacturers, the information on a drug’s patents and regulatory exclusivities is vital for determining when and whether generic competition can occur.12 The requirement for branded companies to list patents in the Orange Book 4 directly enables the “Paragraph IV certification” mechanism and the subsequent “30-month stay” on generic approvals if litigation ensues.38
Understanding Regulatory Exclusivities: A Shield Beyond Patents
Beyond patents, the FDA grants various types of exclusivities upon drug approval, which function as distinct shields of market protection. These exclusivities run for specific durations and, importantly, are not added to the patent life.4
Key types of regulatory exclusivities include:
- New Chemical Entity (NCE) Exclusivity: This provides 5 years of exclusivity and is granted to drugs containing no active moiety that has been previously approved by the FDA.4 It bars the FDA from accepting for review any Abbreviated New Drug Application (ANDA) or 505(b)(2) application for a drug containing the same active moiety during this period.
- Orphan Drug Exclusivity (ODE): This grants 7 years of exclusivity for drugs designated and approved to treat diseases or conditions affecting fewer than 200,000 people in the U.S..2
- “Other” Exclusivity: This provides 3 years of exclusivity for a “change” to an approved drug if the application or supplement contains reports of new clinical investigations (not bioavailability studies) conducted or sponsored by the applicant and deemed essential for approval.4
- Pediatric Exclusivity (PED): This adds 6 months to existing patents and exclusivities.4 It serves as an incentive for companies to conduct studies on the use of their drugs in pediatric populations, ensuring that new treatments are available for all age groups.
- Biologics Price Competition and Innovation Act (BPCIA) Exclusivity: For biological products, this grants 12 years of market exclusivity for reference products, with an additional 1 year for the first interchangeable biosimilar.
A critical distinction for generic manufacturers is that data exclusivity “cannot be legally challenged” , whereas patents are “subject to challenge”. This means that even if a generic company believes a patent is invalid, they may still be prevented from accessing the originator’s clinical trial data for their own applications until the data exclusivity period expires. This creates a different strategic hurdle compared to patent challenges, significantly influencing the timing and viability of generic entry. The ongoing debate around data exclusivity durations, with some advocating for the U.S. to adopt the European Union’s longer 10-11 year periods , highlights the continuous policy tension between incentivizing pharmaceutical innovation (through longer exclusivity) and promoting timely access to affordable medicines (through shorter exclusivity). This dynamic regulatory landscape requires continuous monitoring by business professionals.
Table 3: Specific Types of Exclusivities and Their Durations (US)
| Exclusivity Type | Standard Duration | Key Characteristics |
| New Chemical Entity (NCE) 4 | 5 years | Granted for drugs with no previously approved active moiety; bars FDA from accepting ANDAs for drugs with the same active moiety. |
| Orphan Drug Exclusivity (ODE) 4 | 7 years | Granted for drugs treating rare diseases (affecting <200,000 people in the U.S.). |
| “Other” Exclusivity 4 | 3 years | Granted for new clinical investigations (not bioavailability studies) essential for approval of a change to an approved drug. |
| Pediatric Exclusivity (PED) 4 | 6 months (added to existing IP) | Incentivizes pediatric studies; adds to existing patent or exclusivity terms. |
| 180-Day Generic Exclusivity 4 | 180 days | Granted to the “first” generic applicant to file a Paragraph IV certification and successfully challenge a listed patent. |
| Biologics (BPCIA) | 12 years (reference product) | Market exclusivity for reference biologics, with 1 year for first interchangeable biosimilar. |
The Hatch-Waxman Act: A Game-Changer for Generics
The Drug Price Competition and Patent Term Restoration Act of 1984, commonly known as the Hatch-Waxman Act, fundamentally reshaped the pharmaceutical industry. Its primary purpose was to facilitate the approval of generic drugs while simultaneously providing incentives for innovator companies to continue their research and development efforts.4 The Act streamlined the approval process for generics by allowing Abbreviated New Drug Applications (ANDAs) based on a demonstration of bioequivalence to the brand-name drug, rather than requiring costly and time-consuming full clinical trials.13
Key provisions of the Hatch-Waxman Act include:
- Abbreviated New Drug Application (ANDA): This streamlined pathway allows generic drug manufacturers to seek approval by demonstrating that their product is pharmaceutically equivalent and bioequivalent to a Reference Listed Drug (RLD), which is the brand-name drug it seeks to replicate.6 The ANDA submission must include comprehensive data proving this bioequivalence, along with detailed information about the generic drug’s quality, manufacturing process, and sameness in terms of active ingredients, dosage form, strength, and route of administration.
- Paragraph IV Certification: This is a high-stakes mechanism enabling generic manufacturers to challenge the validity, enforceability, or infringement of a brand-name drug’s listed patents.6 By filing a Paragraph IV certification, a generic company asserts that its proposed product will not infringe any valid, enforceable patent.
- 30-Month Stay: If a branded company files a patent infringement lawsuit within 45 days of receiving a Paragraph IV certification notice, the FDA is required to postpone the generic drug’s approval for 30 months.38 This period allows the patent litigation to proceed and typically expires well before generic entry would otherwise occur. This mechanism creates a structured arena for competition, often leading to significant litigation between innovator and generic companies.
- 180-Day Exclusivity: This serves as a significant incentive granted to the “first” generic applicant to file a substantially complete ANDA containing a Paragraph IV certification and successfully challenge a listed patent.4 This 180-day period of market exclusivity can begin either from the date the first generic begins commercial marketing of the drug or from the date of a court decision favorable to the generic. During this period, the FDA cannot approve subsequently submitted ANDAs for the same drug, even if they are otherwise ready for approval.
The Hatch-Waxman Act simultaneously incentivizes generic entry through streamlined approval and 180-day exclusivity, while providing mechanisms for branded companies to defend their patents through Paragraph IV challenges and the 30-month stay.4 This creates a dynamic, often litigious, environment. Pharmaceutical patent litigation, particularly Paragraph IV challenges, becomes the “critical pathway through which most generic launch timelines are ultimately determined”. Analyzing litigation data, therefore, provides crucial insights into the timing and likelihood of generic entry, elevating legal battles from mere events to primary predictive indicators for market dynamics.
Table 2: Key Provisions of the Hatch-Waxman Act
| Provision | Description | Purpose/Impact |
| Abbreviated New Drug Application (ANDA) 13 | Streamlined approval process for generic drugs, requiring demonstration of bioequivalence to a brand-name drug. | Facilitates faster and less expensive generic entry by avoiding full clinical trials. |
| Paragraph IV Certification 42 | Generic manufacturer certifies that listed patents are invalid or not infringed by their proposed generic product. | High-stakes strategy for early generic market entry; triggers potential litigation. |
| 30-Month Stay 41 | If branded company sues within 45 days of Paragraph IV notice, FDA postpones generic approval for 30 months. | Allows time for patent litigation to resolve; delays generic market entry. |
| 180-Day Exclusivity 42 | Exclusive right to market a generic drug for 180 days, granted to the first Paragraph IV filer who successfully challenges a patent. | Incentivizes early patent challenges; provides significant market advantage to the first generic entrant. |
Mastering the Hunt: Essential Tools and Techniques for Drug Patent Searching
Effective drug patent searching is both an art and a science. It requires not only knowing where to look but also how to craft precise queries and interpret the vast amount of information available. This section outlines the essential tools and sophisticated methodologies that transform basic information retrieval into strategic intelligence gathering.
Publicly Accessible Databases: Your Starting Point
For initial reconnaissance and foundational research, several publicly accessible databases offer a wealth of patent information:
- USPTO Patent Public Search: This is the official and definitive database for U.S. patents and published applications.6 It replaced older legacy tools, offering enhanced access to prior art and supporting a variety of search criteria, including applicant/assignee name, inventor name, publication date ranges, and keywords, with Boolean and proximity operators.45 It also provides access to the Global Dossier, which contains file histories from participating intellectual property (IP) offices, and information on Patent and Trademark Resource Centers (PTRCs) for in-person assistance.
- EPO Espacenet: A comprehensive database from the European Patent Office, Espacenet provides access to millions of patent documents, including worldwide and UK patents.47 It offers technical, legal, and business information, allowing users to track emerging trends and check patent validity.
- WIPO PATENTSCOPE: The World Intellectual Property Organization’s (WIPO) online facility is a global gateway for searching millions of patent documents, including published international Patent Cooperation Treaty (PCT) applications.6 It supports diverse search criteria such as keywords, International Patent Classification (IPC) codes, chemical compounds, and numbers across multiple languages, offering WIPO Translate for instant translation. PATENTSCOPE also includes predefined searches (e.g., for AI or COVID-19 related patents) and provides access to programs like ASPI (Access to Specialized Patent Information) for developing countries.
- Google Patents: A widely used, free patent search engine that indexes patents from various offices globally. It is a convenient tool for quick lookups and general exploration, often serving as a starting point for identifying patent expiration dates.
- I-MAK’s Drug Patent Book: This public resource provides a comprehensive list of patents for top-selling U.S. drugs, detailing patent family grouping, patent type, Orange/Purple Book listing, and a brief description of what each patent covers. It aims to improve transparency and inform policy debates on drug prices.
The widespread availability of robust free databases like USPTO Patent Public Search, Espacenet, and PATENTSCOPE 6 democratizes access to fundamental patent information. This empowers smaller companies, academic researchers, and even the public to conduct initial due diligence and competitive analysis, significantly lowering the barrier to entry for understanding the IP landscape.
Specialized Commercial Platforms: Unlocking Deeper Insights
While public databases offer a solid foundation, specialized commercial platforms provide curated data, advanced analytics, and integrated intelligence capabilities essential for a deeper competitive edge.
- DrugPatentWatch: This platform is a valuable resource offering insights into global drug patents, patent expiration, exclusivity status, and competitive landscapes for pharmaceuticals. It provides data on drug patents, litigation histories, detailed patent and generic drug manufacturer information, and alerts on upcoming patent expirations.53 DrugPatentWatch incorporates data directly from the FDA, USPTO, and other global government sources, updated frequently—often daily—to ensure fresh and accurate information.53 It assists users in identifying market entry opportunities, uncovering prior art in expired and abandoned patents, and tracking first generic entrants. The platform’s focus on providing “insights” and “strategic angles” indicates an emphasis on clear presentation of complex data.30
- Cortellis: A comprehensive platform that combines a vast collection of pharmaceutical industry data and life science content, including news, drug and medical device information, clinical trials, literature, patents, licensing, and company data. It allows for highly flexible patent searches by therapeutic area, company, patent numbers, classification codes, technology, and keywords.
- Derwent Innovation (Clarivate Analytics): This platform provides access to the Derwent World Patents Index (DWPI), a curated patent database featuring editorially enhanced titles and abstracts, specialized subject matter classification coding, and proprietary patent invention-centric family building. Clarivate Patent Intelligence Solutions help organizations evaluate patentability and freedom-to-operate with confidence, and optimize patent portfolios.
- LexisNexis TotalPatent One®: A robust patent search platform offering comprehensive research capabilities across issued patents, published patent applications, utility models, and design patents globally. It boasts over 135 million documents from 107 authorities, including full-text from 67 authorities, with English translations for over 100 million documents. LexisNexis PatentSight® further enhances this with innovation analytics and visualization tools like sunburst charts and advanced patent family charts.
- PatBase (Minesoft/RWS Group): A global patent database covering over 106 authorities, hosting more than 140 million patent and related documents organized into patent families to reduce duplication and save search time. It offers specialized tools like Chemical Explorer for simplifying chemical information searching from patent full text, images, and R-group table compounds.
- PatSnap: This platform connects 116 jurisdictions, 140 million patents, and over 250 million innovation data points from around the world. PatSnap Analytics makes it possible to perform highly targeted global searches that generate reliable, detailed innovation intelligence, helping companies manage risk, spot new opportunities, defend innovations, and monitor the competitive landscape.
- WIPS Global: This service integrates artificial intelligence technology with its system, providing patent analysis services for all levels of users. Its database is optimized for various patent-related tasks, including trial, litigation, generics, chemical compounds, and IP strategy establishment, assisting patent practitioners in efficient performance.
- PatentsKart: This platform offers specialized drug patent linkage databases, including user-friendly and accurate Orange Book and Purple Book data, as well as comprehensive Japanese pharmaceutical patents with their expiration dates. This information is crucial for understanding market exclusivity and potential competition in the Japanese market.
These commercial platforms move beyond simply providing raw data by offering curated data, advanced analytics, and integration with other business intelligence tools.49 This reflects the industry’s need for
actionable intelligence, not just raw data. The ability of these platforms to integrate patent data with other business intelligence sources—such as CRM platforms, market data, and clinical trial information 49—allows for a holistic view of the market. This integrated approach is crucial for comprehensive competitive intelligence and strategic decision-making, providing a significant competitive advantage.
Table 4: Overview of Major Drug Patent Databases
| Database | Type | Primary Focus/Benefits | Key Features |
| USPTO Patent Public Search 6 | Public | Official U.S. patent and application database; enhanced access to prior art. | Boolean/proximity operators, field-specific searching, Global Dossier access. |
| EPO Espacenet 47 | Public | Comprehensive European and worldwide patent documents; technical, legal, business info. | Track emerging trends, check patent validity, millions of documents. |
| WIPO PATENTSCOPE 6 | Public | Global patent search, including PCT applications; multilingual support. | Keywords, IPC, chemical compounds, WIPO Translate, predefined searches. |
| DrugPatentWatch 53 | Commercial | Global drug patent intelligence, expiration, exclusivity, competitive landscapes. | Litigation histories, generic manufacturer info, alerts, market entry opportunities. |
| Cortellis | Commercial | Comprehensive pharma/life science data: news, drugs, trials, literature, patents. | Search by therapeutic area, company, patent numbers, classification codes, keywords. |
| Derwent Innovation 36 | Commercial | Curated patent database (DWPI) with editorial enhancements; patent family building. | Evaluate patentability, FTO, optimize portfolios, extensive data integration. |
| LexisNexis TotalPatent One® 57 | Commercial | Extensive global patent coverage; full-text and English translations. | PatentSight analytics, sunburst charts, advanced patent family charts. |
| PatBase | Commercial | Global patent database organized into patent families; chemical search tools. | Covers 106+ authorities, Chemical Explorer for chemical info searching. |
| PatSnap | Commercial | Connects global patent and innovation data points; targeted searches. | Risk management, opportunity spotting, competitor monitoring, innovation intelligence. |
| WIPS Global | Commercial | AI-integrated patent analysis; optimized for litigation, generics, chemical compounds. | AI technology, specialized databases for patent works, IP strategy. |
| PatentsKart | Commercial | Specialized drug patent linkage databases (Orange/Purple Book, Japan Pharma). | User-friendly search, patent expiration dates, transparency for biologics patents. |
Crafting Your Search Strategy: The Art of Keywords and Operators
Effective patent searching goes beyond simply typing a few words into a search bar. It involves a strategic approach to query construction, leveraging various operators to refine results and ensure comprehensive coverage.
Before diving into databases, it is crucial to define your search scope clearly. This involves identifying the core components of your invention or area of interest, its unique properties, and novel aspects. Using clear and precise language in this initial definition will maximize the effectiveness of your subsequent patent search.
Keyword selection is a critical first step. Think broadly and creatively, including scientific terms, common names, synonyms, and related concepts. Consider different ways an invention might be described.
To refine and expand your search results, a mastery of search operators is essential:
- Boolean Operators:
- AND: Narrows results by requiring all specified terms to be present in the search results (e.g., “drug AND patent”).60
- OR: Expands results by requiring at least one of the specified terms to be present (e.g., “cancer OR oncology”).60
- NOT (or ANDNOT): Excludes terms, refining specificity by removing documents containing the specified term (e.g., “tablet NOT capsule”).60
- Proximity Operators: These operators find terms within a specified number of words from each other (e.g., “drug NEAR/5 delivery”). This is particularly useful when searching for concepts that are frequently mentioned together but not always adjacent, allowing for the capture of relevant documents that might otherwise be missed.60
- Wildcards and Truncation: Symbols such as an asterisk (), question mark (?), or dollar sign ($) can replace one or more characters in a word. This allows for capturing variations, different spellings, or plural forms (e.g., “pharmaceutic” would yield results for pharmaceutical, pharmaceutics, etc.).60
- Phrases: Using quotation marks around a set of words ensures that the search engine finds the exact phrase (e.g., “gene therapy”).
- Field-Specific Searching: Many databases allow users to target searches within specific fields of a patent document, such as the title (.ti.), abstract (.ab.), claims (.clm.), or assignee name (.as.). This granular control enables highly precise analysis and can significantly improve the relevance of search results.
The strategic combination of Boolean, proximity, and truncation operators 60 is not just about finding patents, but about balancing
precision (the relevance of the results) and recall (the completeness of the results). This highlights the iterative and strategic nature of effective patent searching, where the goal is to find all relevant documents without being overwhelmed by irrelevant ones.
Table 5: Common Patent Search Operators and Their Use
| Operator | Function | Example | Impact on Results |
| Boolean Operators | |||
| AND 60 | Requires all terms to be present. | drug AND delivery | Narrows results, increases precision. |
| OR 60 | Requires at least one term to be present. | cancer OR oncology | Expands results, increases recall. |
| NOT (or ANDNOT) 60 | Excludes specific terms. | tablet NOT capsule | Narrows results, refines specificity. |
| Proximity Operators | |||
| NEAR (e.g., NEAR/5) 60 | Finds terms within a specified distance of each other. | “drug” NEAR/5 “formulation” | Finds related concepts mentioned closely, improving relevance. |
| Wildcards/Truncation | |||
| * (asterisk) 60 | Replaces one or more characters at the end of a word. | pharmaceutic* | Finds pharmaceutical, pharmaceutics, etc., capturing variations. |
| ? (question mark) | Replaces a single character. | color? | Finds color, colour, etc., capturing spelling variations. |
| Phrases | |||
| “” (quotation marks) | Searches for an exact phrase. | “gene therapy” | Ensures terms appear together in the specified order. |
Navigating Classification Systems: IPC, CPC, and USPC
Beyond keywords, patent classification systems offer a powerful, structured approach to searching, especially when dealing with complex technical fields or international documents.
Patent classification systems—such as the International Patent Classification (IPC), Cooperative Patent Classification (CPC), and US Patent Classification (USPC)—categorize patents based on their technical features. This systematic categorization makes it significantly easier to find relevant prior art in specific technological areas. These systems provide language-independent symbols for classification, which is a key advantage for global patent searching.62
- IPC (International Patent Classification): Established by the Strasbourg Agreement, the IPC is a hierarchical system of language-independent symbols used globally for classifying patents and utility models according to different areas of technology.62 WIPO provides various resources, including IPCCAT (a categorization assistance tool) and STATS (a tool for IPC predictions based on statistical analysis), to assist users with IPC classification and searching.
- CPC (Cooperative Patent Classification): Jointly managed by the European Patent Office (EPO) and the USPTO, the CPC is an extension of the IPC, offering a finer subdivision with approximately 250,000 entries.61 It is largely compatible with the IPC, and CPC codes can often be easily converted to their hierarchically higher IPC equivalents.
- USPC (US Patent Classification): This is the classification system historically used by the USPTO. While still relevant for older U.S. patents, the CPC is increasingly becoming the standard for cooperative classification.
Leveraging classification codes involves identifying the primary classification codes relevant to your invention and using them to search within patent databases. Cross-references within these systems can help uncover related categories that might not be immediately obvious from keyword searches. The increasing harmonization of classification systems, such as the CPC, indicates a clear trend toward greater standardization and transparency in patent information worldwide.
The use of classification systems, with their language-independent symbols 62, is a powerful tool for overcoming language barriers in global patent searching. This provides a critical advantage over keyword-only searches, which can be limited by translation nuances and terminology differences. Furthermore, leveraging classification codes is a strategic method for assessing novelty (ensuring an invention is truly new) and conducting Freedom-to-Operate (FTO) analyses (ensuring a product does not infringe existing patents). By categorizing patents by technical features, classification systems allow for a highly targeted and comprehensive review of the technological landscape, directly supporting these critical business decisions.
Table 6: Essential INID Codes for Drug Patent Documents
| INID Code | Description | Significance for Drug Patents |
| (11) 27 | Number of the patent document (publication number) | Unique identifier for the patent; essential for direct lookup. |
| (21) 27 | Number given to the application (application number) | Identifies the specific patent application. |
| (22) 27 | Date of making application (filing date) | Crucial for determining the 20-year patent term and priority. |
| (31) 27 | Number assigned to priority application | Links to earlier applications from which priority is claimed. |
| (32) 27 | Date of filing of priority application (priority date) | The earliest date of invention, critical for prior art assessment. |
| (33) | Country in which priority application was filed | Indicates the origin of the priority claim. |
| (43) 27 | Publication day of unexamined application | Date the patent application became publicly available (often 18 months from priority). |
| (45) | Date of publication by printing of a granted patent | Date the patent was officially granted. |
| (51) | International Patent Classification (IPC) | Indicates the primary technical classification of the invention. |
| (54) 27 | Title of the invention | Provides a concise overview of the patent’s subject matter. |
| (57) 27 | Abstract or claim | Summarizes the invention and its scope. |
| (71) 27 | Name of applicant | Identifies the current owner of the patent application. |
| (72) 27 | Name of inventor | Identifies the individual(s) who conceived the invention. |
The Chemical Code: Searching by Structure (SMILES, Markush)
For chemical and pharmaceutical inventions, searching by chemical structure is often far more precise and effective than keyword searching alone. Chemical compounds can have multiple names, complex descriptions, or subtle structural variations that keyword searches might miss entirely.68
Chemical structure representations form the basis of these searches. Queries typically begin with either a graphical depiction (e.g., molecular formulas, skeletal structures, 2D/3D models) or a textual notation (e.g., SMILES notation, Mol files).
- SMILES Notation: The Simplified Molecular Input Line Entry System (SMILES) is a textual representation of chemical structures that allows for clear and concise description of molecules.36 AI-driven platforms like PatSight can automate the extraction of key data from chemical patents and deliver results in formats like CSV or SDF files with SMILES notation.
- Markush Structures: These are powerful, generic representations used extensively in patent claims to define a broad class or family of related chemical compounds.68 Markush structures combine both visual and textual elements to describe a molecular backbone with variable substituents at specific positions.70 A single Markush formula allows inventors to claim a wide variety of similar compounds in one claim, effectively preventing competitors from making minor molecular modifications to bypass the patent. Some Markush structures can be so general that they encompass millions of actual chemical entities.71
Search algorithms and matching criteria for chemical structure searches rely on specialized algorithms and databases capable of understanding and interpreting these complex chemical structures, including Markush structures. Users can specify matching criteria such as exact match, substructure match (finding records containing a specified structural fragment), or a similarity threshold.
Specialized databases for chemical structure search, such as CAS Scientific Patent Explorer, allow for direct searching of patents by chemical structure, including Markush searches. Commercial databases like STN (which includes CASLINK, REGISTRY, MARPAT, and CAPLUS) are also crucial for this type of detailed chemical patent searching.
Chemical structure searching, especially with Markush structures, is paramount for chemical and pharmaceutical patents. It allows for highly precise prior art searches (to determine novelty) and infringement analyses (to ensure freedom-to-operate), as it accounts for the vast number of chemical variations that keyword searches alone might miss.69 The unique nature of chemical claims necessitates this specialized approach. Furthermore, the increasing role of AI in chemical structure recognition, with platforms like PatSight automating “accurate chemical structure recognition” and tools like MarkushGrapher using multimodal approaches to interpret complex Markush structures , signifies a significant technological advancement. AI is making complex chemical patent searching more efficient and accurate, enhancing the capabilities of patent intelligence.
Beyond the Surface: Interpreting Patent Documents for Strategic Advantage
Raw patent data is merely the starting point. The true value lies in interpreting these complex documents to extract strategic intelligence. This section delves into the nuances of patent claims, specifications, legal statuses, and prosecution histories, transforming the reader into a patent intelligence maestro.
Deconstructing Patent Claims: The Heart of Protection
Patent claims are the legal statements that define the precise scope of an invention’s protection. They are typically written as a single, heavily punctuated sentence and appear towards the end of the patent document. These claims represent the legal boundaries of what the patent owner can exclude others from doing.
- Independent Claims: These are the broadest claims in a patent, standing alone without referring to any other claim. They define the core invention in its most expansive terms, providing the inventor with the most comprehensive protection.16
- Example: “A composition, comprising a solvent, and a metal salt dissolved in the solvent.”
- Dependent Claims: These claims refer back to and incorporate all the limitations of a preceding independent or dependent claim, adding further specific features or limitations to narrow the scope of protection.16 They serve as crucial fallback positions in case broader claims are challenged or invalidated, offering more specific protection that might be upheld.
- Example: “The composition of claim 1, further comprising a pH-modifying substrate in an amount sufficient to adjust the pH to a value of from 3.5-5.”.
The transitional phrases used in claims, such as “comprising,” “consisting essentially of,” and “consisting of,” are pivotal in defining the claim’s scope.
- “Comprising” is an open-ended term, meaning the invention includes the recited elements but may also include other unrecited elements. This provides the broadest scope.
- “Consisting essentially of” represents a middle ground. It means the invention includes the recited elements and may include others, but only if those others do not materially affect the basic and novel properties of the claimed invention. The interpretation of this phrase can be highly nuanced and heavily influenced by the patent’s prosecution history, as illustrated in the Eye Therapies case. In that case, the Federal Circuit determined that “consisting essentially of” meant brimonidine as the sole active ingredient, based on arguments made during prosecution to distinguish the invention from prior art. This demonstrates how the precise choice of transitional phrases directly impacts the scope of protection and can be the deciding factor in patent infringement litigation.
- “Consisting of” is a closed-ended term, meaning the invention includes only the recited elements, making it the narrowest in scope.
Claim interpretation typically accords claims their plain and ordinary meaning, but an inventor has the right to act as their own lexicographer to define terms within the patent. The prosecution history, which documents the entire examination process, can significantly influence this interpretation, particularly when terms have an “atypical meaning” or when the scope of claims is ambiguous. Courts often refer to this history to understand the inventor’s intent and how claims were narrowed or defined during examination.
Drafting strong patent claims for drug inventions is a highly strategic endeavor, often likened to “sculpting a masterpiece”. It demands a deep understanding of existing prior art, adherence to legal precepts, and strategic foresight to create a “resilient and protective shield” for the drug invention. This process is not merely a technical exercise but a critical strategic one that directly impacts the defensibility and commercial value of a pharmaceutical innovation.
Unpacking the Specification: Unveiling the Invention’s Secrets
While claims define the legal boundaries, the specification (or detailed description) provides the technical blueprint of the invention. This section offers a thorough description, including the chemical structure, synthesis methods, therapeutic uses, formulation details, and pharmacological properties of the drug. It is within the specification that the “bargain” of public disclosure is fulfilled, requiring the inventor to provide enough detail for others to understand and replicate the invention once the patent term expires. This is known as the “enablement” requirement in patent law, ensuring that the public benefits from the disclosed knowledge, even if it aids future competitors.
Synthesis methods are frequently described in detail within patents, ranging from general overviews to step-by-step procedures. These descriptions often include crucial information on starting materials, reaction conditions, purification techniques, and strategies for yield optimization. The level of detail provided in the patent specification regarding synthesis methods directly impacts the ease with which generic manufacturers can “design around” existing patents or develop their own non-infringing processes. While fulfilling disclosure requirements, a thorough specification can inadvertently provide a roadmap for competitors seeking to enter the market.
The specification may also contain experimental data, presented as “working examples” (describing actual performed work or achieved results) or “prophetic examples” (describing predicted or simulated results). Prophetic examples, sometimes called “paper” or “hypothetical” examples, are included to demonstrate the invention’s applicability and support the breadth of the claims, even if the experiments have not yet been performed. For pharmaceutical inventions, some data demonstrating a substantial likelihood of the invention working as a human pharmaceutical is typically required.
Strategic analysis of the specification involves more than just reading; it means identifying the active ingredients, recognizing potential modifications, and planning alternative synthesis routes. This granular level of detail enables precise analysis of patent claims, helping to understand the exact scope of protection, identify potential vulnerabilities, and track ownership, which is crucial for assessing patent strength, potential infringement risks, and strategic competitive positioning.
Understanding Patent Legal Statuses: Active, Expired, Litigated, and More
A patent’s legal status is a dynamic indicator of its enforceability, market exclusivity, and susceptibility to generic competition. Continuous monitoring of these statuses is crucial for accurate competitive intelligence and risk assessment.
- Active/Granted/In Force: This status indicates that the patent is currently valid and enforceable, providing the patent holder with exclusive rights.
- Pending: The patent application has been published or is under examination, but the patent has not yet been issued or formally abandoned.
- Expired: The patent term has ended, and the protection it afforded has ceased.2 This status is a green light for generic competition, as it opens the door for other manufacturers to produce and sell their versions of the drug.
- Abandoned: A patent application or granted patent is no longer active, often due to the applicant’s failure to respond to office actions within specified timeframes or to pay required maintenance fees.74 In some circumstances, an abandoned application may be revived.
- Withdrawn: An application that was open to public inspection was formally withdrawn at the request of the applicant.
- Rejected: An application has been rejected by the patent examiner during the prosecution phase.
- Revoked/Invalidated: The patent has been declared invalid, either partially or wholly. This often occurs through opposition proceedings initiated by a third party or as a result of litigation.
- Lapsed/Ceased: This status often results from the non-payment of annual maintenance fees, leading to the invalidation of the patent.
- Litigated: The patent is currently involved in a legal dispute, such as an infringement suit or a Paragraph IV challenge.17 This is a key indicator for potential generic entry and requires close monitoring, as the outcome directly influences market timelines.
- Re-examined: A third party or the inventor has requested a re-examination of the patent to verify its patentability.
- Opposed: A third party has initiated an opposition proceeding to challenge the validity of a pending patent application or a granted patent.
- Pledged/Trust: These statuses indicate financial arrangements where the patent rights are used as collateral, similar to a mortgage.
Patents involved in litigation, particularly those subject to Paragraph IV challenges, are critical indicators for predicting generic drug launches. The outcome of this litigation directly dictates when a generic can enter the market, potentially even through “at-risk” launches where a generic manufacturer begins selling its product before patent litigation concludes, accepting the risk of potential damages if they are ultimately found to infringe. This makes litigation status a primary predictive variable for understanding market dynamics. A patent’s “active” status is not static; it can be challenged, abandoned, or invalidated. This dynamic nature means continuous monitoring of legal status is essential for accurate competitive intelligence and risk assessment, as the current legal vulnerability or strength of a patent must be continuously assessed.
The Power of Prosecution History: What Happened Behind the Scenes?
The prosecution history refers to the complete record of communications and actions between the patent applicant and the patent office during the examination process. This includes all office actions (rejections, objections), the applicant’s responses, any amendments made to the claims or specification, and the arguments presented to overcome rejections.
The significance of prosecution history is profound, particularly for interpreting patent claims. While claims are typically given their plain and ordinary meaning, the prosecution history can reveal instances where terms were given an “atypical meaning” or where the scope of claims was narrowed or defined during examination. Courts frequently refer to this history to understand the inventor’s intent and the precise scope of protection that was ultimately granted.
A compelling case example is Eye Therapies v. Slayback Pharma. In this case, the Federal Circuit Court of Appeals used the prosecution history of the ‘742 patent to interpret the transitional phrase “consisting essentially of” in the claims. During prosecution, Eye Therapies had amended its claims from “comprising” to “consisting essentially of” to distinguish its invention from prior art that disclosed combination therapies. The examiner, in allowing the claims, noted that this amendment meant the claimed methods “do not require the use of any other active ingredients in addition to brimonidine”. The court ultimately relied on this prosecution history to conclude that the phrase “consisting essentially of” in this specific context meant brimonidine as the sole active ingredient, thereby excluding other active ingredients from the scope of the claims. This case vividly illustrates how statements and amendments made during patent prosecution can significantly impact the interpretation of claim terms in subsequent litigation, potentially altering the scope of patent protection.
The prosecution history reveals the negotiation between the applicant and the patent office. It is not just about what was ultimately granted, but why it was granted and what scope was surrendered or clarified along the way. This provides invaluable context for assessing a patent’s true strength and potential vulnerabilities, offering a deeper understanding of its defensibility or susceptibility to challenges beyond its face value.
Turning Data into Gold: Strategic Applications of Patent Intelligence
For business professionals, patent data is not merely information; it is a strategic asset. Sophisticated analysis of patent intelligence can drive competitive advantage across various facets of the pharmaceutical business, from R&D to market entry and risk mitigation.
Competitive Intelligence: Tracking R&D Pipelines and Market Shifts
Patent intelligence serves as a powerful early warning system for competitive threats. By systematically monitoring and analyzing patent filings, companies can gain unprecedented insights into competitor research directions, technological innovations, and potential market entries years before products reach the market. This allows organizations to make informed decisions about their own R&D investments, potentially redirecting resources to more promising or less crowded therapeutic areas.
- Pipeline Monitoring: Tracking patent applications provides a window into competitor R&D pipelines, revealing their priorities, emerging therapeutic areas, and product development.36 For instance, the filing of initial composition of matter patents might indicate early-stage programs that will require development support in 1-2 years, while formulation patents might suggest programs approaching clinical development and commercial manufacturing. This allows for proactive rather than reactive R&D adjustments.
- Technology Trend Identification: Patent activity signals shifts in target selection within therapeutic areas, emerging modalities gaining traction (e.g., from small molecules to biologics), evolving approaches to addressing specific mechanisms of action, changing formulation and delivery technologies, and new manufacturing processes being adopted.
- Mapping Competitor Platforms: Analyzing patents helps identify core molecular scaffolds or biological platforms, enabling technologies that span multiple products, and cross-therapeutic applications of similar approaches that form a competitor’s underlying technology platform.
- Strategic Decision-Making: Patent intelligence enables the evaluation of the potential impact of competitor innovations on existing product lines, the assessment of freedom-to-operate constraints for pipeline products, the identification of patent challenges or invalidation opportunities, and the development of contingency plans for market entry by competitive products.36
By leveraging patent intelligence, companies can shift from a reactive stance—merely responding to competitor launches—to a proactive one, anticipating market shifts and competitive moves. This is the essence of competitive advantage, enabling strategic maneuvering before competitors even realize a threat exists.
Freedom-to-Operate (FTO) Analysis: Mitigating Infringement Risks
Freedom-to-Operate (FTO) analysis, also known as a “clearance search,” is a critical due diligence process undertaken to ensure that a new product, process, or service does not infringe on existing, active patents held by others.
The process involves a comprehensive search of patent databases for in-force patents that might be relevant to a company’s product or technology. This includes a meticulous analysis of patent claims, their legal status, and the geographic coverage of the patents. Given the extensive “patent thickets” that often surround many drugs, FTO analysis is particularly vital in the pharmaceutical industry to prevent costly legal disputes that can halt product launches and incur massive damages.78
Identifying potential infringement risks early in the development lifecycle allows companies to implement crucial risk mitigation strategies. These can include “designing around” existing patents by modifying the product or process, negotiating licensing agreements with patent holders, or proactively challenging problematic patents through legal avenues.24 A robust FTO analysis directly impacts a company’s ability to bring a product to market without legal encumbrances. Failure to conduct thorough FTO can lead to expensive litigation, injunctions that block market entry, and significant financial penalties.78
Market Entry Strategies: Seizing Generic Opportunities
For generic pharmaceutical manufacturers, timing is everything when it comes to market entry. Entering too early risks costly patent infringement litigation, while entering too late means missing the crucial “first-to-file” advantage.
- Leveraging Orange Book Data: Analyzing patent expiration dates and regulatory exclusivity periods listed in the FDA’s Orange Book is fundamental for identifying optimal market entry windows for generic or biosimilar products.13 DrugPatentWatch, with its constantly updated insights on patent expiration, is a key tool for this analysis.
- Paragraph IV Challenges: Generic companies can strategically challenge the validity or non-infringement of innovator patents via Paragraph IV certifications.39 If successful, the first generic applicant to file such a certification is eligible for 180 days of market exclusivity, preventing other generics from entering during that period and allowing them to capture a significant market share.4 This incentivizes early patent challenges and accelerates generic competition. The 180-day exclusivity granted to the first generic filer directly leads to a significant market share advantage (typically 70-80% within the first year) and higher initial prices for that first entrant.39 This creates a high-stakes “race” for generic companies.
- Predicting Generic Launches: Tracking litigation data, including “at-risk” launches (where a generic manufacturer begins selling its product before patent litigation concludes, taking a calculated risk), is crucial for predicting generic entry timelines. The first generic entrant typically prices its products 15-30% below the brand, and within 12 months, multiple generics usually enter, driving prices down 50-80%. Brand products often lose 80-90% of market share within one year of multiple generic entry.
The impending expiration of patents on blockbuster drugs, often referred to as the “patent cliff” 37, is not merely a loss for branded companies but a significant catalyst for market transformation. This phenomenon creates massive opportunities for generic manufacturers and fundamentally shifts the overall market landscape.13 Strategic responses by innovator companies facing generic entry include lowering their prices, launching authorized generics, or accelerating the development of next-generation products to mitigate anticipated revenue loss.37
Identifying White Space: Fueling Future Innovation
Beyond defensive strategies, patent intelligence is a powerful tool for identifying “white space”—therapeutic areas, technological approaches, or delivery mechanisms with limited or no existing patent coverage. These areas often indicate unmet needs or untapped market opportunities.
The methodology for identifying white space involves a comprehensive patent landscape analysis. This includes mapping all patents within a therapeutic area of interest, analyzing citation patterns to find highly influential early-stage technologies, and identifying shifts in technology trends. For example, a company might discover a mechanistically related target with minimal patent activity despite promising biology, or identify delivery approaches not yet claimed for specific indications.
The strategic value of identifying white space is immense. It allows companies to redirect R&D investments to more promising or less crowded areas, fostering genuine breakthrough innovation rather than incremental improvements. It can also reveal opportunities for novel applications of existing technologies or combination therapies with minimal competitive activity. The identification of these patent gaps directly influences R&D prioritization, leading companies to invest in truly novel areas rather than competing in crowded fields.
Navigating “Evergreening” Strategies: Challenges and Countermeasures
“Evergreening” is a controversial strategy employed by pharmaceutical companies to extend the lifetime of their patent monopolies. This is often achieved by obtaining numerous additional patents on minor variations of an original drug, such as new formulations, dosage forms, routes of administration, or new uses.7 The primary aim of evergreening is to prolong monopoly periods and delay generic drug entry, thereby maintaining high drug costs.
Common types of evergreening include:
- Product Hopping/Switching: This involves patenting minor variations like changes to the drug’s physical form (e.g., capsule to tablet), subtle chemical changes (e.g., chiral switches to different enantiomeric mixtures), or switching from short-acting to long-acting formulations.23
- New Formulations/Delivery Methods: Patenting extended-release versions or novel delivery systems for an existing drug.9
- New Methods of Use/Indications: Securing patents for novel therapeutic uses of an existing drug, even if the drug compound itself is old.2
- Patent Clustering/Thickets: Building a “comprehensive ‘web of protection'” by accumulating multiple patents around a single drug, making it difficult for generics to navigate the IP landscape without infringement.7
The impact of evergreening can be substantial. For instance, product hopping of the multiple sclerosis drug glatiramer acetate (Copaxone) resulted in an estimated cost to consumers of $4.3 to $6.5 billion over two and a half years. This illustrates that evergreening transforms what might seem like a niche legal strategy into a major public health and economic issue. It also undermines the fundamental “patent bargain” by extending monopolies beyond what might be considered genuine innovation.
While secondary patents can protect genuine follow-on innovation, evergreening highlights how the patent system can be “strategically manipulated” to prolong monopolies and delay generic entry.7 Distinguishing between these two motivations is “very difficult” , creating a significant point of controversy and policy debate.
For generic manufacturers, understanding these strategies is crucial for developing effective countermeasures:
- Paragraph IV Challenges: Directly challenging the validity or non-infringement of evergreening patents is a primary legal avenue.6
- “Skinny Labels”: Marketing a generic for only the non-patented uses of a drug, thereby “carving out” patented method-of-use indications, allows generics to avoid infringement.6
- Developing Novel Synthesis Routes: Generic companies can invest in R&D to devise manufacturing processes that avoid patented methods of synthesis.
- Litigation: Engaging in legal battles to invalidate or bypass these secondary patents is a common strategy to accelerate generic market entry.7
Table 7: Common Evergreening Strategies
| Strategy | Description | Example | Impact |
| Product Hopping/Switching 23 | Patenting minor variations (e.g., dosage form, chemical changes, release characteristics) of an existing drug. | Switching from short- to long-acting formulations; chiral switches (e.g., Copaxone, Namenda).23 | Extends market exclusivity, delays generic entry, can cost consumers billions. |
| New Formulations/Delivery Methods 9 | Patenting new ways a drug is prepared or administered. | Extended-release versions (e.g., Prozac, Glucophage XR) or novel delivery systems. | Prolongs patent protection, enhances patient compliance or efficacy. |
| New Methods of Use/Indications 2 | Securing patents for novel therapeutic uses of an existing drug. | Discovering a new disease indication for an already approved drug. | Expands market scope, creates new revenue streams, extends exclusivity for that specific use. |
| Patent Clustering/Thickets 7 | Accumulating numerous patents around a single drug to create a complex web of protection. | Multiple patents covering different aspects: compound, formulation, process, use, polymorphs. | Deters generic entry by increasing litigation risk and complexity for challengers. |
Table 8: Pharmaceutical Patent Litigation Statistics (US)
| Statistic | Value/Trend (2023) | Implication |
| % of patent infringement cases resulting in injunctions | 45% | Injunctions are a significant remedy for patent holders to stop ongoing infringement. |
| % of patent cases with preliminary injunctions granted | 30% | Courts view certain infringement scenarios with urgency, granting temporary halts early in litigation. |
| % of patent infringement cases with permanent injunctions issued | 20% | Permanent injunctions are less common but represent a definitive, long-term halt to infringing activities. |
| % of injunctions granted in favor of the plaintiff | 60% | Patent holders (plaintiffs) have a higher likelihood of securing injunctions to protect their IP. |
| Median duration for a preliminary injunction | 6 months | Provides a temporary but significant period to prevent further harm while the case proceeds. |
| Median duration for a permanent injunction | 18 months | Long-term impact on infringing parties, effectively stopping activities related to the patented invention. |
| % of all patent injunctions related to pharmaceutical patents | 15% | Highlights the heavy reliance on patent protection and litigation in the pharmaceutical industry. |
The Horizon of Innovation: Emerging Trends in Patent Intelligence
The landscape of patent intelligence is continuously evolving, driven by technological advancements that are revolutionizing how patent data is searched, analyzed, and leveraged.
The Rise of AI and Machine Learning in Patent Analysis
Artificial Intelligence (AI) and Machine Learning (ML) are transforming patent analysis, moving beyond traditional manual processes to enable unprecedented speed, depth, and predictive capabilities.
- Automated Search and Analysis: AI algorithms can rapidly analyze vast amounts of patent documents, identifying relevant prior art, potential threats, and weaknesses in IP portfolios. They automate routine tasks such as preliminary searches, freeing up patent professionals to focus on more complex analyses that require human expertise and judgment. This ability to analyze scores of documents quickly and automate repetitive tasks directly leads to increased speed and depth in patent analysis, allowing for more comprehensive and timely competitive intelligence.
- Predictive Capabilities: AI tools can predict future trends in technology and patent filings, offering forward-looking foresights that enable proactive strategic planning.76 Machine learning algorithms can even predict optimal reaction conditions, potential side reactions, and yield improvements in drug synthesis, further streamlining R&D efforts.
- Enhanced Data Integration: AI and big data analytics facilitate the integration of multiple data sources, including patent databases, scientific literature, and market data, for more thorough and comprehensive patent searches.
- Specific AI Applications:
- Chemical Structure Recognition: AI-driven platforms like PatSight automate the extraction of key data from chemical patents, including accurate chemical structure recognition and the automated identification of structures, names, and biological activity data.
- Markush Structure Analysis: AI tools such as MarkushGrapher employ multimodal approaches that integrate both textual and visual data to recognize and interpret complex Markush structures, translating them into searchable formats like CXSMILES.
- Semantic Analysis: Advanced AI techniques enable concept extraction, similarity analysis (grouping related patents regardless of terminology differences), and sentiment analysis (detecting emphasis on specific advantages or problems), and tracking how the description of technologies changes over time.
The impact of AI is profound: it significantly streamlines the patent search process, extracts deeper and more nuanced information, and enables faster, more efficient evaluation of an invention’s novelty, technical feasibility, and commercial value. While AI automates many tasks, the role of human patent professionals shifts from manual searching to focusing on more complex analyses and interpreting the AI-generated insights. This suggests a future of human-AI collaboration, where AI enhances, rather than replaces, expert judgment, requiring new skill sets in data interpretation and the strategic application of AI-generated intelligence.
Data Visualization: Making Complex Landscapes Comprehensible
The sheer volume and complexity of patent data can be overwhelming. Data visualization tools are emerging as indispensable assets for transforming intricate intellectual property domains into intuitive, comprehensible visual formats.83 This transformation is crucial for translating dense textual datasets into actionable strategic intelligence.
- Transforming Complexity: Pharma data visualization cuts through noisy data to reveal the connections and patterns necessary for informed decision-making. It reduces complexity, allowing decision-makers to accurately identify competitive threats, emerging technology trends, and white spaces ripe for innovation. This directly addresses the human cognitive limitation in processing vast amounts of textual data.
- Types of Visualizations: These tools offer a variety of visual representations, including graphical dashboards, heat maps, interactive data visualizations, sunburst charts (for quickly understanding the makeup of a patent portfolio), and advanced patent family charts (for digesting a wide range of patent insights simultaneously).58
- Strategic Benefits:
- Identifying Trends: Visualization allows for quick identification of technology trends, emerging areas, and innovation trajectories.47
- Competitive Positioning: It provides a clear overview of the intellectual property landscape, untangling connections between patents, classifications, assignees, and inventors.
- White Space Identification: Visual tools help pinpoint patent clusters, technology hotspots, and “white spaces ripe for innovation”.
- Decision Support: By visualizing both the current state and potential evolution of technology domains, these tools empower decision-makers to quickly identify valuable innovation avenues, understand shifts in competitive dynamics, and mitigate infringement risks.
- Integration with Workflows: Powerful visualizations can be integrated into existing workflows, enabling high-quality biomarker identification processes and unified analysis across multiple research sites, as seen in large pharmaceutical companies like Roche.
DrugPatentWatch aligns with broader business intelligence trends that combine business analytics, data mining, data visualization, and infrastructure to help organizations make more data-driven decisions. While specific visualization tools are not detailed for DrugPatentWatch, its focus on providing “insights” and enabling exploration of “multiple strategic angles” suggests a strong emphasis on the clear and effective presentation of complex data.30
Effective data visualization enables “strategic storytelling” , where complex patent portfolios can be presented to stakeholders in an intuitive way, facilitating understanding of strengths, weaknesses, and strategic directions. This goes beyond mere presentation; it enables a more compelling and understandable narrative around complex IP data, which is crucial for influencing strategic decisions within an organization.
Table 9: AI/ML Tools and Their Applications in Patent Intelligence
| AI/ML Application | Description | Benefits |
| Advanced Search & Analysis | Automates analysis of vast patent documents; identifies prior art, threats, IP weaknesses. | Increased speed and depth; frees professionals for complex tasks. |
| Predictive Analytics 76 | Forecasts future technology trends and patent filings. | Provides forward-looking foresights for proactive strategic planning. |
| Chemical Structure Recognition | Automates extraction and identification of chemical structures, names, and biological activity data from patents. | Enhances precision and efficiency in chemical patent searching. |
| Markush Structure Analysis | Interprets complex Markush structures using multimodal data (textual & visual). | Enables comprehensive searching of broad chemical classes defined in patents. |
| Semantic Analysis | Extracts concepts, groups related patents by similarity, tracks terminology evolution. | Uncovers hidden connections and trends regardless of keyword variations. |
| Data Integration | Combines patent data with scientific literature, market data, and other sources. | Provides more thorough and comprehensive patent intelligence. |
Key Takeaways
The strategic landscape of the pharmaceutical industry is inextricably linked to the dynamics of drug patents. For business and pharmaceutical professionals, leveraging patent data is not merely an optional exercise but a fundamental driver of competitive advantage.
- Drug patents are the economic bedrock of pharmaceutical innovation, incentivizing billions in R&D investment by granting temporary market monopolies.
- A clear understanding of the distinction between patents and regulatory exclusivities (such as NCE, ODE, and 180-day generic exclusivity) is paramount for accurate market forecasting, as these distinct protections dictate the true timelines for market entry.
- The Hatch-Waxman Act established a dynamic, often litigious, framework that both facilitates generic entry and provides mechanisms for branded companies to defend their intellectual property. Consequently, litigation data has become a critical predictor of generic drug launches.
- Mastering advanced patent search techniques—extending beyond simple keyword searches to include Boolean operators, proximity searches, classification systems (IPC, CPC), and critically, chemical structure searching (SMILES, Markush structures)—is essential for comprehensive and precise intelligence gathering in the pharmaceutical domain.
- Interpreting patent documents requires a deep dive beyond the abstract, necessitating the deconstruction of claims (understanding the impact of transitional phrases) and a thorough analysis of the specification (for synthesis methods and experimental data) to uncover the true scope, strengths, and vulnerabilities of a patent.
- Patent intelligence serves as a proactive competitive advantage tool, enabling companies to monitor competitor R&D pipelines, identify emerging technologies, conduct robust Freedom-to-Operate (FTO) analyses to mitigate infringement risks, and pinpoint white space opportunities for future innovation.
- Understanding and navigating “evergreening” strategies is crucial for both branded companies (to legitimately extend product lifecycles) and generic manufacturers (to identify and challenge potentially anti-competitive practices).
- The future of patent intelligence is being redefined by the transformative power of Artificial Intelligence and advanced data visualization, which convert complex data into digestible, actionable intelligence, enabling a more efficient, predictive, and human-augmented analytical process.
- DrugPatentWatch stands out as a valuable specialized platform that integrates diverse data sources, offering constantly updated insights on patent expiration, litigation, and generic entry, which are crucial for informed decision-making in drug lifecycle management.
Frequently Asked Questions (FAQ)
1. How does the “effective patent life” of a drug differ from its statutory 20-year term, and why does this matter for business strategy?
The statutory patent term is generally 20 years from the filing date of the patent application.1 However, pharmaceutical companies often file patents very early in the drug development process to secure priority and attract investment, long before clinical trials begin or regulatory approval is obtained.2 This means a significant portion of the 20-year term is consumed during the lengthy R&D and regulatory review phases. Consequently, the “effective patent life”—the period during which the drug enjoys market exclusivity
after FDA approval—is often much shorter, typically ranging from 7 to 12 years.3 This distinction is crucial for business strategy because it dictates the actual window for recouping R&D investments and maximizing profits before generic competition enters the market.2 Understanding this shorter effective life drives strategies like evergreening and the pursuit of patent term extensions to prolong market exclusivity.
2. What is the strategic significance of “Paragraph IV Certification” under the Hatch-Waxman Act for both branded and generic pharmaceutical companies?
Paragraph IV Certification is a pivotal mechanism under the Hatch-Waxman Act where a generic drug manufacturer notifies the FDA and the brand-name drug patent holder that they believe the listed patents are either invalid or will not be infringed by their proposed generic product.6
- For Branded Companies: It serves as an alert to potential generic competition and triggers a critical decision point. If they file an infringement lawsuit within 45 days of receiving the notice, it automatically imposes a 30-month stay on the FDA’s approval of the generic application, buying the branded company time to litigate and maintain market exclusivity.38
- For Generic Companies: It is a high-stakes strategy to gain early market entry. If successful in challenging the patent, the first generic applicant to file a Paragraph IV certification is eligible for 180 days of market exclusivity, preventing other generics from entering during that period and allowing them to capture a significant market share.4 This incentivizes early patent challenges and accelerates generic competition.
3. How do specialized commercial patent databases like DrugPatentWatch enhance competitive intelligence beyond what public databases offer?
While public databases (e.g., USPTO, EPO, WIPO PATENTSCOPE) provide foundational access to patent documents 6, specialized commercial platforms like DrugPatentWatch offer significantly enhanced capabilities crucial for competitive intelligence.53
- Curated and Integrated Data: DrugPatentWatch, for instance, integrates data directly from multiple government sources (FDA, USPTO, foreign governments) and other critical information like litigation histories, generic manufacturer details, and clinical trials.53 This goes beyond raw patent data to provide a holistic view.
- Actionable Intelligence and Analytics: These platforms provide “constantly-updated insights on drug patent expiration, generic entry opportunities, and critical litigation updates”. They offer tools for tracking global patent statuses, analyzing competition, identifying market opportunities, and generating alerts on upcoming patent expirations, which is instrumental for developing strategic approaches to product development, market entry, and patent strategy.
- Advanced Features: Many commercial platforms incorporate AI and machine learning for automated analysis, predictive capabilities, and specialized chemical structure recognition (e.g., Markush structures), transforming complex data into digestible, actionable intelligence.36 This enables companies to conduct their own analyses tuned to their specific strategic needs, more efficiently identifying invalidity opportunities, anticipating generic entry windows, and navigating patent roadblocks than through traditional analytical intermediaries.
4. What are “evergreening” strategies, and why are they a point of contention in the pharmaceutical industry?
“Evergreening” refers to various legal and business strategies by which pharmaceutical companies extend the lifetime of their patent monopolies on existing drugs that are nearing expiration.7 This is often achieved by obtaining numerous additional patents on minor variations of the original drug, such as new formulations, dosage forms, routes of administration, or new therapeutic uses.7
Evergreening is a point of contention because it delays the entry of generic medicines, which are typically much more affordable, leading to higher costs for patients and taxpayers.32 Critics argue that this practice artificially extends market monopolies and undermines the “patent bargain”—the trade-off where inventors receive temporary exclusivity in exchange for public disclosure that eventually allows for competition. While some secondary patents may protect genuine follow-on innovation, distinguishing this from strategic efforts to prolong monopolies with limited additional inventiveness is often difficult.31 The economic impact can be substantial; for example, product hopping of a multiple sclerosis drug reportedly cost consumers billions of dollars over a few years. This highlights the ongoing tension between incentivizing pharmaceutical innovation and ensuring timely access to affordable medicines.
5. How do AI and data visualization tools enhance the strategic value derived from drug patent searching?
AI and data visualization tools significantly enhance the strategic value of drug patent searching by transforming the efficiency, depth, and comprehensibility of patent analysis.81
- AI’s Contribution: AI algorithms can rapidly analyze vast amounts of patent documents, automating routine tasks like preliminary searches and extracting deeper insights that would be challenging for human analysts alone.81 This includes advanced chemical structure recognition (e.g., SMILES, Markush structures), predictive capabilities for technology trends, and semantic analysis to group related patents.36 AI streamlines the entire process, enabling faster and more efficient evaluation of novelty, technical feasibility, and commercial value.
- Data Visualization’s Contribution: Patent landscape visualization systematically processes patent data into intuitive visual formats, such as graphical dashboards, heat maps, and interactive charts.83 This capability cuts through the complexity of dense textual datasets, making intricate IP domains comprehensible for decision-makers.83 It allows for quick identification of emerging technology trends, competitive positioning, and “white spaces” ripe for innovation.47 Essentially, visualization enables “strategic storytelling” with data, allowing stakeholders to quickly grasp strengths, weaknesses, and strategic directions within complex patent portfolios.
Together, AI and data visualization empower businesses to move from reactive responses to proactive strategic planning, leveraging patent intelligence to gain a significant competitive edge in the dynamic pharmaceutical market.
References
- www.drugpatentwatch.com, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/how-long-do-drug-patents-last/#:~:text=Patents%20serve%20as%20a%20critical,date%20of%20patent%20application%20filing.
- Drug Patents: How Pharmaceutical IP Incentivizes Innovation and Affects Pricing, accessed July 24, 2025, https://www.als.net/news/drug-patents/
- Drug Patents and Generic Pharmaceutical Drugs – News-Medical.net, accessed July 24, 2025, https://www.news-medical.net/health/Drug-Patents-and-Generics.aspx
- Patents and Exclusivity | FDA, accessed July 24, 2025, https://www.fda.gov/media/92548/download
- Pharmaceutical Industry Facts & Figures – IFPMA, accessed July 24, 2025, https://www.ifpma.org/wp-content/uploads/2025/02/IFPMA_Always_Innovating_Facts__Figures_Report.pdf
- Decode a Drug Patent Like a Wall Street Analyst – DrugPatentWatch, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/how-to-check-if-a-drug-is-patented/
- The Role of Patents in Drug Innovation and Generic Competition, accessed July 24, 2025, https://jchr.org/index.php/JCHR/article/view/8396
- What are the types of pharmaceutical patents? – Patsnap Synapse, accessed July 24, 2025, https://synapse.patsnap.com/blog/what-are-the-types-of-pharmaceutical-patents
- Filing Strategies for Maximizing Pharma Patents: A Comprehensive Guide for Business Professionals – DrugPatentWatch, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/filing-strategies-for-maximizing-pharma-patents/
- Drug Patent Life: The Complete Guide to Pharmaceutical Patent Duration and Market Exclusivity – DrugPatentWatch, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/how-long-do-drug-patents-last/
- The Benefits From Giving Makers Of Conventional `Small Molecule’ Drugs Longer Exclusivity Over Clinical Trial Data, accessed July 24, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC3804334/
- Patent Listing in FDA’s Orange Book – Congress.gov, accessed July 24, 2025, https://www.congress.gov/crs-product/IF12644
- Generic Drug Entry Timeline: Predicting Market Dynamics After Patent Loss, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/generic-drug-entry-timeline-predicting-market-dynamics-after-patent-loss/
- How to Draft Patent Claims for Chemical Inventions – PatentPC, accessed July 24, 2025, https://patentpc.com/blog/how-to-draft-patent-claims-for-chemical-inventions
- PATENT CLAIM FORMAT AND TYPES OF CLAIMS – WIPO, accessed July 24, 2025, https://www.wipo.int/edocs/mdocs/aspac/en/wipo_ip_phl_16/wipo_ip_phl_16_t5.pdf
- Introduction to Patent Claims – Fish & Richardson, accessed July 24, 2025, https://www.fr.com/insights/ip-law-essentials/introduction-to-patent-claims/
- What is the difference between a composition claim and a method of use claim?, accessed July 24, 2025, https://wysebridge.com/what-is-the-difference-between-a-composition-claim-and-a-method-of-use-claim
- Understanding Independent and Dependent Claims in Patents – YouTube, accessed July 24, 2025, https://www.youtube.com/watch?v=hfschitCLDU
- Dependent Claims – WIPO, accessed July 24, 2025, https://www.wipo.int/edocs/mdocs/aspac/en/wipo_ip_mnl_3_18/wipo_ip_mnl_3_18_p_5.pdf
- How Dependent Patent Claims add Value: A Case in Point – Dilworth IP, accessed July 24, 2025, https://www.dilworthip.com/resources/news/dependent-claims/
- Prosecution History Can Inform an Atypical … – Duane Morris LLP, accessed July 24, 2025, https://www.duanemorris.com/alerts/prosecution_history_can_inform_atypical_meaning_patent_claim_term_0725.html
- How to Draft Strong Patent Claims for Drug Inventions – PatentPC, accessed July 24, 2025, https://patentpc.com/blog/how-to-draft-strong-patent-claims-drug-inventions
- Patent protection strategies – PMC, accessed July 24, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC3146086/
- Using drug patent information to synthesize drugs – DrugPatentWatch, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/using-drug-patent-information-to-synthesize-drugs/
- Use of prophetic examples in patent specifications – Spruson & Ferguson, accessed July 24, 2025, https://www.spruson.com/use-of-prophetic-examples-in-patent-specifications/
- How to Conduct a Patent Search for Chemical Inventions – PatentPC, accessed July 24, 2025, https://patentpc.com/blog/how-to-conduct-a-patent-search-for-chemical-inventions
- Patent document INID codes and how they can help you | epo.org, accessed July 24, 2025, https://www.epo.org/en/searching-for-patents/helpful-resources/patent-knowledge-news/patent-document-inid-codes-and-how
- INID Codes – Numbers Identifying Bibliographic Data in Patent Documents or in Patent Gazettes, accessed July 24, 2025, http://mpasearch.co.uk/inid-codes
- Types of Pharmaceutical Patent – Product, Process, Formulation and Method, accessed July 24, 2025, http://mpasearch.co.uk/product-process-formulation-patents
- DrugPatentWatch Analysis: Patenting Drug Combinations Requires Focusing on Unique, Combined Benefits – GeneOnline News, accessed July 24, 2025, https://www.geneonline.com/drugpatentwatch-analysis-patenting-drug-combinations-requires-focusing-on-unique-combined-benefits/
- cdip_15_inf_2.docx – WIPO, accessed July 24, 2025, https://www.wipo.int/edocs/mdocs/mdocs/en/cdip_15/cdip_15_inf_2.docx
- Pharmaceutical Patents and Evergreening** – The Australian National University, accessed July 24, 2025, https://researchportalplus.anu.edu.au/en/publications/pharmaceutical-patents-and-evergreening
- Evergreening – Wikipedia, accessed July 24, 2025, https://en.wikipedia.org/wiki/Evergreening
- What is a priority date? – OC Patent Lawyer, accessed July 24, 2025, https://ocpatentlawyer.com/what-is-a-priority-date/
- Understanding Priority Claims for U.S. Patent Applications: Part 1 – Mintz, accessed July 24, 2025, https://www.mintz.com/insights-center/viewpoints/2231/2018-07-22-understanding-priority-claims-us-patent-applications
- How to Track Competitor R&D Pipelines Through Drug Patent Filings, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/how-to-track-competitor-rd-pipelines-through-drug-patent-filings/
- The Impact of Patent Expirations on Generic Drug Market Entry – PatentPC, accessed July 24, 2025, https://patentpc.com/blog/the-impact-of-patent-expirations-on-generic-drug-market-entry
- Orange Book Listable? | Insights & Resources – Goodwin, accessed July 24, 2025, https://www.goodwinlaw.com/en/insights/blogs/2020/10/orange-book-listable
- The Strategic Value of Orange Book Data in Pharmaceutical Competitive Intelligence, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/the-strategic-value-of-orange-book-data-in-pharmaceutical-competitive-intelligence/
- Drug Patent Linkage Databases – Patentskart, accessed July 24, 2025, https://patentskart.com/drug-patent-linkage-database/
- The timing of 30‐month stay expirations and generic entry: A cohort study of first generics, 2013–2020 – PMC, accessed July 24, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8504843/
- Small Business Assistance | 180-Day Generic Drug Exclusivity – FDA, accessed July 24, 2025, https://www.fda.gov/drugs/cder-small-business-industry-assistance-sbia/small-business-assistance-180-day-generic-drug-exclusivity
- Understanding Patent Term Extensions in Different Countries – PatentPC, accessed July 24, 2025, https://patentpc.com/blog/understanding-patent-term-extensions-in-different-countries
- The Role of Litigation Data in Predicting Generic Drug Launches – DrugPatentWatch, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/the-role-of-litigation-data-in-predicting-generic-drug-launches/
- Search for patents – USPTO, accessed July 24, 2025, https://www.uspto.gov/patents/search
- Patent public search FAQs – USPTO, accessed July 24, 2025, https://www.uspto.gov/patents/search/patent-public-search/faqs
- Searching for patents | epo.org, accessed July 24, 2025, https://www.epo.org/en/searching-for-patents
- Search for a patent – GOV.UK, accessed July 24, 2025, https://www.gov.uk/search-for-patent
- How CDMOs Can Use Patent Data to Win More Pharmaceutical Clients – DrugPatentWatch, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/how-cdmos-can-use-patent-data-to-win-more-pharmaceutical-clients/
- Patents – World Health Organization (WHO), accessed July 24, 2025, https://www.who.int/observatories/global-observatory-on-health-research-and-development/resources/databases/databases-on-processes-for-r-d/patents
- PATENTSCOPE – WIPO, accessed July 24, 2025, https://www.wipo.int/en/web/patentscope
- Drug Patent Book Background & Methods – I-MAK, accessed July 24, 2025, https://www.i-mak.org/patent-methods/
- Find Your Next Blockbuster – Biotech & Pharmaceutical patents, sales, drug prices, litigation – DrugPatentWatch, accessed July 24, 2025, https://www.drugpatentwatch.com/about.php
- DrugPatentWatch | Software Reviews & Alternatives – Crozdesk, accessed July 24, 2025, https://crozdesk.com/software/drugpatentwatch
- Transforming Biopharma Intelligence: Moving from Traditional Analysts to Direct Raw Data Platforms – DrugPatentWatch, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/transforming-biopharma-intelligence-moving-from-traditional-analysts-to-direct-raw-data-platforms/
- Patent search: Cortellis – Baker Library – Harvard Business School, accessed July 24, 2025, https://www.library.hbs.edu/services/help-center/patent-search-cortellis
- ASPI – Specialized Patent Information – WIPO, accessed July 24, 2025, https://www.wipo.int/en/web/aspi
- Must-Have Patent Visualization Tools – LexisNexis IP, accessed July 24, 2025, https://www.lexisnexisip.com/resources/must-have-patent-visualization-tools/
- A Complete Guide To Pharmaceutical Competitive Intelligence – WatchMyCompetitor, accessed July 24, 2025, https://www.watchmycompetitor.com/resources/a-complete-guide-to-pharmaceutical-competitive-intelligence/
- Patent Search Strategies and Techniques – WIPO, accessed July 24, 2025, https://www.wipo.int/edocs/mdocs/africa/en/wipo_tiscs_znz_16/wipo_tiscs_znz_16_t_6.pdf
- Advanced Patent Search Techniques for Experienced Researchers …, accessed July 24, 2025, https://patentpc.com/blog/advanced-patent-search-techniques-for-experienced-researchers
- International Patent Classification (IPC) – WIPO, accessed July 24, 2025, https://www.wipo.int/en/web/classification-ipc
- ST.9 – Recommendation concerning bibliographic data on and relating to patents and SPCs, accessed July 24, 2025, https://www.cnipa.gov.cn/module/download/down.jsp?i_ID=181896&colID=3160
- What are INID numbers and why are they important in patent documents? – BlueIron IP, accessed July 24, 2025, https://blueironip.com/ufaqs/what-are-inid-numbers-and-why-are-they-important-in-patent-documents/
- IPC and CPC Basics – WIPO, accessed July 24, 2025, https://www.wipo.int/edocs/mdocs/africa/en/wipo_ip_pre_16/wipo_ip_pre_16_t_8.pdf
- Cooperative Patent Classification (CPC) | epo.org, accessed July 24, 2025, https://www.epo.org/en/searching-for-patents/helpful-resources/first-time-here/classification/cpc
- Search Patent Classification Systems – USPTO, accessed July 24, 2025, https://www.uspto.gov/web/patents/classification/
- Chemical Structure Patent Search – CAS Scientific Patent Explorer Help, accessed July 24, 2025, https://help.patentexplorer.cas.org/cur/Searching_in_Patent_Explorer/Patent_Search/Find_Patents_by_Chemical_Structure.htm
- Chemical Structure Search – Effectual Services, accessed July 24, 2025, https://www.effectualservices.com/patent-services/patent-searches/chemical-structure-search/
- [Literature Review] MarkushGrapher: Joint Visual and Textual Recognition of Markush Structures – Moonlight | AI Colleague for Research Papers, accessed July 24, 2025, https://www.themoonlight.io/en/review/markushgrapher-joint-visual-and-textual-recognition-of-markush-structures
- US20050010603A1 – Display for Markush chemical structures – Google Patents, accessed July 24, 2025, https://patents.google.com/patent/US20050010603A1/en
- Markush searches simplified | CAS, accessed July 24, 2025, https://www.cas.org/resources/gated-content/markush-searches-simplified
- What are Markush structures, and how can I draw them – ACD/Labs, accessed July 24, 2025, https://www.acdlabs.com/blog/markush-structures-what-they-are-and-how-to-draw-them/
- Simple Legal Status / Legal Status / Legal Event Search Guide – Patsnap Help Center, accessed July 24, 2025, https://help.patsnap.com/hc/en-us/articles/115005282709-Simple-Legal-Status-Legal-Status-Legal-Event-Search-Guide
- 711-Abandonment of Patent Application – USPTO, accessed July 24, 2025, https://www.uspto.gov/web/offices/pac/mpep/s711.html
- Pharmaceutical Competitive Intelligence | 2025 Guide – BiopharmaVantage, accessed July 24, 2025, https://www.biopharmavantage.com/competitive-intelligence
- Patent Intelligence – IQVIA, accessed July 24, 2025, https://www.iqvia.com/solutions/commercialization/commercial-analytics-and-consulting/brand-strategy-and-management/patent-intelligence
- Patent Injunction Statistics: Trends and Implications – PatentPC, accessed July 24, 2025, https://patentpc.com/blog/patent-injunction-statistics-trends-and-implications
- Life Sciences & Pharma IP Litigation 2025 – USA – Global Practice Guides, accessed July 24, 2025, https://practiceguides.chambers.com/practice-guides/life-sciences-pharma-ip-litigation-2025/usa/trends-and-developments
- Navigating the Patent Cliff: Strategies for Excipient Business Growth Through Patent Intelligence – DrugPatentWatch – Transform Data into Market Domination, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/grow-your-excipient-business-by-tracking-drug-patents/
- The Future of Patent Intelligence Tools: How AI is Revolutionizing the Landscape, accessed July 24, 2025, https://www.drugpatentwatch.com/blog/the-future-of-patent-intelligence-tools-how-ai-is-revolutionizing-the-landscape/
- Relecura – Where AI makes a difference in your innovation landscape, accessed July 24, 2025, https://relecura.ai/
- Use Cases for Pharma Data Visualization – Cambridge Intelligence, accessed July 24, 2025, https://cambridge-intelligence.com/use-cases/pharma/
- Patent Landscape Visualization: Intellectual Property Analysis Tools : r/AnalyticsAutomation, accessed July 24, 2025, https://www.reddit.com/r/AnalyticsAutomation/comments/1llgk8b/patent_landscape_visualization_intellectual/
- Patent Term Extension Statistics: What Innovators Need to Know – PatentPC, accessed July 24, 2025, https://patentpc.com/blog/patent-term-extension-statistics-what-innovators-need-to-know
- Data exclusivity | European Medicines Agency (EMA), accessed July 24, 2025, https://www.ema.europa.eu/en/glossary-terms/data-exclusivity
- 716-Affidavits or Declarations Under 37 CFR 1.132 and Other Evidence Traversing Rejections – USPTO, accessed July 24, 2025, https://www.uspto.gov/web/offices/pac/mpep/s716.html


























